The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice
- PMID: 12391329
- PMCID: PMC524320
- DOI: 10.1073/pnas.212498399
The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice
Abstract
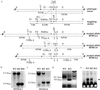
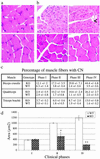
Myotubularin is a ubiquitously expressed phosphatase that acts on phosphatidylinositol 3-monophosphate [PI(3)P], a lipid implicated in intracellular vesicle trafficking and autophagy. It is encoded by the MTM1 gene, which is mutated in X-linked myotubular myopathy (XLMTM), a muscular disorder characterized by generalized hypotonia and muscle weakness at birth leading to early death of most affected males. The disease was proposed to result from an arrest in myogenesis, as the skeletal muscle from patients contains hypotrophic fibers with centrally located nuclei that resemble fetal myotubes. To understand the physiopathological mechanism of XLMTM, we have generated mice lacking myotubularin by homologous recombination. These mice are viable, but their lifespan is severely reduced. They develop a generalized and progressive myopathy starting at around 4 weeks of age, with amyotrophy and accumulation of central nuclei in skeletal muscle fibers leading to death at 6-14 weeks. Contrary to expectations, we show that muscle differentiation in knockout mice occurs normally. We provide evidence that fibers with centralized myonuclei originate mainly from a structural maintenance defect affecting myotubularin-deficient muscle rather than a regenerative process. In addition, we demonstrate, through a conditional gene-targeting approach, that skeletal muscle is the primary target of murine XLMTM pathology. These mutant mice represent animal models for the human disease and will be a valuable tool for understanding the physiological role of myotubularin.
Figures




References
-
- Buj-Bello A., Biancalana, V., Moutou, C., Laporte, J. & Mandel, J. L. (1999) Hum. Mutat. 14, 320-325. - PubMed
-
- Herman G. E., Finegold, M., Zhao, W., de Gouyon, B. & Metzenberg, A. (1999) J. Pediatr. 134, 206-214. - PubMed
-
- Laporte J., Biancalana, V., Tanner, S. M., Kress, W., Schneider, V., Wallgren-Pettersson, C., Herger, F., Buj-Bello, A., Blondeau, F., Liechti-Gallati, S. & Mandel, J. L. (2000) Hum. Mutat. 15, 393-409. - PubMed
-
- Fardeau M., (1992) Skeletal Muscle Pathology (Churchill Livingstone, Edinburgh).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

