Inositol pyrophosphates regulate endocytic trafficking
- PMID: 12391334
- PMCID: PMC137862
- DOI: 10.1073/pnas.212527899
Inositol pyrophosphates regulate endocytic trafficking
Abstract
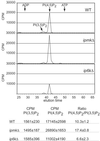
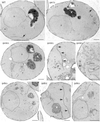
The high energy potential and rapid turnover of the recently discovered inositol pyrophosphates, such as diphosphoinositol-pentakisphosphate and bis-diphosphoinositol-tetrakisphosphate, suggest a dynamic cellular role, but no specific functions have yet been established. Using several yeast mutants with defects in inositol phosphate metabolism, we identify dramatic membrane defects selectively associated with deficient formation of inositol pyrophosphates. We show that this phenotype reflects specific abnormalities in endocytic pathways and not other components of membrane trafficking. Thus, inositol pyrophosphates are major regulators of endocytosis.
Figures


References
-
- Stephens L., Radenberg, T., Thiel, U., Vogel, G., Khoo, K. H., Dell, A., Jackson, T. R., Hawkins, P. T. & Mayr, G. W. (1993) J. Biol. Chem. 268, 4009-4015. - PubMed
-
- Menniti F. S., Miller, R. N., Putney, J. W., Jr. & Shears, S. B. (1993) J. Biol. Chem. 268, 3850-3856. - PubMed
-
- Shears S. B., Ali, N., Craxton, A. & Bembenek, M. E. (1995) J. Biol. Chem. 270, 10489-10497. - PubMed
-
- Saiardi A., Caffrey, J. J., Snyder, S. H. & Shears, S. B. (2000) J. Biol. Chem. 275, 24686-24692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

