Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice
- PMID: 12393844
- PMCID: PMC150794
- DOI: 10.1172/JCI15623
Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice
Abstract
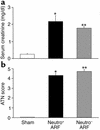
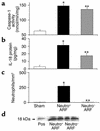
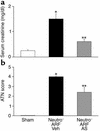
Having recently described the injurious role of caspase-1-mediated production of the proinflammatory cytokine IL-18 in ischemic acute renal failure (ARF), we report here on the effect of the newly developed caspase inhibitor Quinoline-Val-Asp(Ome)-CH(2)-OPH (OPH-001) on caspase-1, IL-18, neutrophil infiltration, and renal function in ischemic ARF. C57BL/6 mice with ischemic ARF treated with OPH-001 had a marked (100%) reduction in blood urea nitrogen (BUN) and serum creatinine and a highly significant reduction in morphological acute tubular necrosis (ATN) score compared with vehicle-treated mice. OPH-001 significantly reduced the increase in caspase-1 activity and IL-18 and prevented neutrophil infiltration in the kidney during ischemic ARF. To evaluate whether this lack of neutrophil infiltration was contributing to the protection against ischemic ARF, a model of neutrophil depletion was developed. Neutrophil-depleted mice had a small (18%) reduction in serum creatinine during ischemic ARF but no reduction in ATN score despite a lack of neutrophil infiltration in the kidney. Remarkably, caspase-1 activity and IL-18 were significantly increased in the kidney in neutrophil-depleted mice with ARF. In addition, IL-18 antiserum-treated neutrophil-depleted mice with ischemic ARF had a significant (75%) reduction in serum creatinine and a significant reduction in ATN score compared with vehicle-treated neutrophil-depleted mice. These results suggest a novel neutrophil-independent mechanism of IL-18-mediated ischemic ARF.
Figures
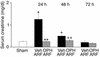


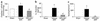
References
-
- Kaushal GP, Ueda N, Shah SV. Role of caspases (ICE/CED 3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int. 1997;52:438–445. - PubMed
-
- Edelstein CL, Shi Y, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J Am Soc Nephrol. 1999;10:1940–1949. - PubMed
-
- Kaushal GP, Singh AB, Shah SV. Identification of gene family of caspases in rat kidney and altered expression in ischemia reperfusion injury. Am J Physiol. 1998;274:F587–F595. - PubMed
-
- Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Down-regulation of the calpain inhibitor protein, calpastatin, by caspases during renal ischemia. Am J Physiol. 2000;279:F509–F517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

