Laboratory response to anthrax bioterrorism, New York City, 2001
- PMID: 12396923
- PMCID: PMC2730291
- DOI: 10.3201/eid0810.020376
Laboratory response to anthrax bioterrorism, New York City, 2001
Abstract
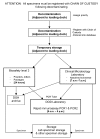
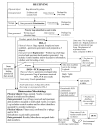
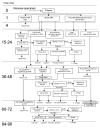
In October 2001, the greater New York City Metropolitan Area was the scene of a bioterrorism attack. The scale of the public response to this attack was not foreseen and threatened to overwhelm the Bioterrorism Response Laboratory's (BTRL) ability to process and test environmental samples. In a joint effort with the Centers for Disease Control and Prevention and the cooperation of the Department of Defense, a massive effort was launched to maintain and sustain the laboratory response and return test results in a timely fashion. This effort was largely successful. The development and expansion of the facility are described, as are the special needs of a BTRL. The establishment of a Laboratory Bioterrorism Command Center and protocols for sample intake, processing, reporting, security, testing, staffing, and and quality control are also described.
Figures

References
-
- Centers for Disease Control and Prevention. Update: Investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001. MMWR Morb Mortal Wkly Rep. 2001;50:909–19. - PubMed
-
- Richmond JY, McKinney RW, eds. Biosafety in microbiological and biomedical laboratories. HHS pub. no. CDC 93-8395, 3rd edition. Washington: U.S. Department of Health and Human Services Public Health Service, Centers for Disease Control and Prevention and National Institutes of Health; 1993.
-
- Centers for Disease Control and Prevention. Public health emergency preparedness and response: agents of bioterrorism. CDC:PHEPR 2002. Available from: URL: http://www.bt.cdc.gov/labissues/index.asp
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
