The PrpC serine-threonine phosphatase and PrkC kinase have opposing physiological roles in stationary-phase Bacillus subtilis cells
- PMID: 12399479
- PMCID: PMC151969
- DOI: 10.1128/JB.184.22.6109-6114.2002
The PrpC serine-threonine phosphatase and PrkC kinase have opposing physiological roles in stationary-phase Bacillus subtilis cells
Abstract
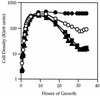
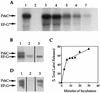
Loss of the PrpC serine-threonine phosphatase and the associated PrkC kinase of Bacillus subtilis were shown to have opposite effects on stationary-phase physiology by differentially affecting cell density, cell viability, and accumulation of beta-galactosidase from a general stress reporter fusion. These pleiotropic effects suggest that PrpC and PrkC have important regulatory roles in stationary-phase cells. Elongation factor G (EF-G) was identified as one possible target of the PrpC and PrkC pair in vivo, and purified PrpC and PrkC manifested the predicted phosphatase and kinase activities against EF-G in vitro.
Figures

References
-
- Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165-177. - PubMed
-
- Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Völker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

