A proline-rich region with a highly periodic sequence in Streptococcal beta protein adopts the polyproline II structure and is exposed on the bacterial surface
- PMID: 12399508
- PMCID: PMC151936
- DOI: 10.1128/JB.184.22.6376-6393.2002
A proline-rich region with a highly periodic sequence in Streptococcal beta protein adopts the polyproline II structure and is exposed on the bacterial surface
Abstract
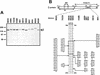
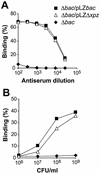
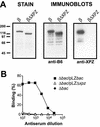
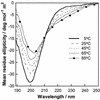
Proline-rich regions have been identified in many surface proteins of pathogenic streptococci and staphylococci. These regions have been suggested to be located in cell wall-spanning domains and/or to be required for surface expression of the protein. Because little is known about these regions, which are found in extensively studied and biologically important surface proteins, we characterized the proline-rich region in one such protein, the beta protein of group B streptococci. The proline-rich region in beta, designated the XPZ region, has a proline at every third position, and the sequence is highly periodic in other respects. Immunochemical analysis showed that the XPZ region was not associated with the cell wall but was exposed on the bacterial surface. Moreover, characterization of a beta mutant lacking the XPZ region demonstrated that this region was not required for surface expression of the beta protein. Comparison of the XPZ region in different beta proteins showed that it varied in size but always retained the typical sequence periodicity. Circular dichroism spectroscopy indicated that the XPZ region had the structure of a polyproline II helix, an extended and solvent-exposed structure with exactly three residues per turn. Because of the three-residue sequence periodicity in the XPZ region, it is expected to be amphipathic and to have distinct nonpolar and polar surfaces. This study identified a proline-rich structure with unique properties that is exposed on the surface of an important human pathogen.
Figures
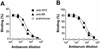
References
-
- Adzhubei, A. A., and M. J. E. Sternberg. 1993. Left-handed polyproline II helices commonly occur in globular proteins. J. Mol. Biol. 229:472-493. - PubMed
-
- Anthonyraj, K. J., T. Karunakaran, and P. A. Raj. 1998. Bactericidal activity and poly-L-proline II conformation of the tandem repeat sequence of human salivary mucin glycoprotein (MG2). Arch. Biochem. Biophys. 356:197-206. - PubMed
-
- Areschoug, T., M. Stålhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal β protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 277:12642-12648. - PubMed
-
- Arnott, S., and S. D. Dover. 1968. The structure of poly-L-proline II. Acta Crystallogr. B 24:599-601. - PubMed
-
- Baker, C. J., and M. S. Edwards. 1995. Group B streptococcal infection, p. 980-1054. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and the newborn infant. W. B. Saunders Company, Philadelphia, Pa.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

