Elevated c-Src is linked to altered cell-matrix adhesion rather than proliferation in KM12C human colorectal cancer cells
- PMID: 12402152
- PMCID: PMC2376185
- DOI: 10.1038/sj.bjc.6600594
Elevated c-Src is linked to altered cell-matrix adhesion rather than proliferation in KM12C human colorectal cancer cells
Abstract
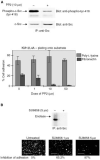
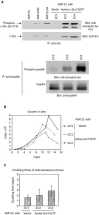
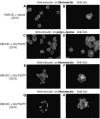
Elevated expression and/or activity of c-Src, the prototype of the Src family of protein tyrosine kinases, is associated with the development of human colon cancer. However, despite the known pleiotropic effects of these kinases in promoting (a) cell growth downstream of growth factor receptors, and (b) the dynamic regulation of integrin adhesions in fibroblast model systems, their precise role in epithelial cancer cells is unknown. Here we addressed whether elevated expression and activity of cellular Src alters cell proliferation and/or cell-matrix adhesion in cancer cells from the Fidler model of colorectal metastasis. Although elevated Src correlates with ability to metastasise to the liver after intrasplenic injection, we found that this was not linked to enhanced growth, either in vitro or in vivo as sub-cutaneous tumours. However, elevated Src was associated with enhanced attachment to extracellular matrix. In addition, adhesion to fibronectin, was suppressed by agents that inhibited Src activity, while enforced elevation of Src in non-metastatic cells was sufficient to stimulate adhesion to fibronectin and enhanced assembly of adhesion complexes, without influencing cell growth. Thus, we conclude that one role of elevated Src in human colon cancer cells is to modulate integrin-dependent cell-matrix attachment and formation of adhesion structures, which may, in turn, influence cell motility and integrin-dependent cellular responses.
Copyright 2002 Cancer Research UK
Figures



Similar articles
-
SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling.Mol Cell Biol. 2004 Sep;24(18):8113-33. doi: 10.1128/MCB.24.18.8113-8133.2004. Mol Cell Biol. 2004. PMID: 15340073 Free PMC article.
-
Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling.Nat Cell Biol. 2002 Aug;4(8):632-8. doi: 10.1038/ncb829. Nat Cell Biol. 2002. PMID: 12134161
-
Transformation of chicken embryo fibroblasts by v-src uncouples beta1 integrin-mediated outside-in but not inside-out signaling.Mol Cell Biol. 2001 Nov;21(21):7295-306. doi: 10.1128/MCB.21.21.7295-7306.2001. Mol Cell Biol. 2001. PMID: 11585912 Free PMC article.
-
The interplay between Src and integrins in normal and tumor biology.Oncogene. 2004 Oct 18;23(48):7928-46. doi: 10.1038/sj.onc.1208080. Oncogene. 2004. PMID: 15489911 Review.
-
v-Src's hold over actin and cell adhesions.Nat Rev Mol Cell Biol. 2002 Apr;3(4):233-45. doi: 10.1038/nrm779. Nat Rev Mol Cell Biol. 2002. PMID: 11994743 Review.
Cited by
-
The SRC family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy.Clin Colorectal Cancer. 2010 Apr;9(2):89-94. doi: 10.3816/CCC.2010.n.012. Clin Colorectal Cancer. 2010. PMID: 20378502 Free PMC article. Review.
-
Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial-mesenchymal transition.Mol Biol Cell. 2004 Jun;15(6):2794-803. doi: 10.1091/mbc.e03-12-0879. Epub 2004 Apr 9. Mol Biol Cell. 2004. PMID: 15075377 Free PMC article.
-
Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530.Mol Oncol. 2009 Jun;3(3):248-61. doi: 10.1016/j.molonc.2009.01.002. Epub 2009 Feb 7. Mol Oncol. 2009. PMID: 19393585 Free PMC article.
-
Adhesion signaling by a novel mitotic substrate of src kinases.Oncogene. 2005 Aug 11;24(34):5333-43. doi: 10.1038/sj.onc.1208582. Oncogene. 2005. PMID: 16007225 Free PMC article.
-
Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells.Mol Cancer Res. 2008 Nov;6(11):1766-74. doi: 10.1158/1541-7786.MCR-08-0169. Mol Cancer Res. 2008. PMID: 19010823 Free PMC article.
References
-
- AbramCLCourtneidgeSA2000Src family tyrosine kinases and growth factor signaling Exp Cell Res 254113 - PubMed
-
- AvizienyteEWykeAWJonesRJMcLeanGWWesthoffMABruntonVGFrameMC2002Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling Nat Cell Biol 4632638 - PubMed
-
- BaroneMVCourtneidgeSA1995Myc but not Fos rescue of PDGF signalling block caused by kinase- inactive Src Nature 378509512 - PubMed
-
- BolenJBVeilletteASchwartzAMDeseauVRosenN1987Analysis of pp60c-src in human colon carcinoma and normal human colon mucosal cells Oncogene Res 1149168 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

