Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3
- PMID: 12403815
- PMCID: PMC2173047
- DOI: 10.1083/jcb.200203074
Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3
Abstract
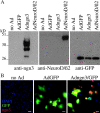
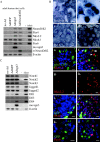
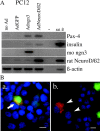
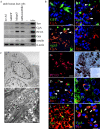
Regulatory proteins have been identified in embryonic development of the endocrine pancreas. It is unknown whether these factors can also play a role in the formation of pancreatic endocrine cells from postnatal nonendocrine cells. The present study demonstrates that adult human pancreatic duct cells can be converted into insulin-expressing cells after ectopic, adenovirus-mediated expression of the class B basic helix-loop-helix factor neurogenin 3 (ngn3), which is a critical factor in embryogenesis of the mouse endocrine pancreas. Infection with adenovirus ngn3 (Adngn3) induced gene and/or protein expression of NeuroD/beta2, Pax4, Nkx2.2, Pax6, and Nkx6.1, all known to be essential for beta-cell differentiation in mouse embryos. Expression of ngn3 in adult human duct cells induced Notch ligands Dll1 and Dll4 and neuroendocrine- and beta-cell-specific markers: it increased the percentage of synaptophysin- and insulin-positive cells 15-fold in ngn3-infected versus control cells. Infection with NeuroD/beta2 (a downstream target of ngn3) induced similar effects. These data indicate that the Delta-Notch pathway, which controls embryonic development of the mouse endocrine pancreas, can also operate in adult human duct cells driving them to a neuroendocrine phenotype with the formation of insulin-expressing cells.
Figures

References
-
- Apelqvist, A., H. Li, L. Sommer, P. Beatus, D.J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature. 400:877–881. - PubMed
-
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. - PubMed
-
- Assady, S., G. Maor, M. Amit, J. Itskovitz-Eldor, K.L. Skorecki, and M. Tzukerman. 2001. Insulin production by human embryonic stem cells. Diabetes. 50:1691–1697. - PubMed
-
- Atouf, F., P. Czernichow, and R. Scharfmann. 1997. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J. Biol. Chem. 272:1929–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

