Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts
- PMID: 12405967
- PMCID: PMC3281755
- DOI: 10.1046/j.1460-9568.2002.02224.x
Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts
Abstract
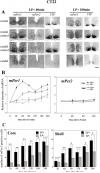
The mechanism whereby brief light exposure resets the mammalian circadian clock in a phase dependent manner is not known, but is thought to involve Per gene expression. At the behavioural level, a light pulse produces phase delays in early subjective night, phase advances in late subjective night, and no phase shifts in mid-subjective night or subjective day. To understand the relationship between Per gene activity and behavioural phase shifts, we examined light-induced mPer1 and mPer2 expression in the suprachiasmatic nucleus (SCN) of the mouse, in the subjective night, with a view to understanding SCN heterogeneity. In the VIP-containing region of the SCN (termed 'core'), light-induced mPer1 expression occurs at all times of the subjective night, while mPer2 induction is seen only in early subjective night. In the remaining regions of the SCN (termed 'shell'), a phase delaying light pulse produces no mPer1 but significant mPer2 expression, while a phase advancing light pulse produces no mPer2 but substantial mPer1 induction. Moreover, following a light pulse during mid-subjective night, neither mPer1 nor mPer2 are induced in the shell. The results reveal that behavioural phase shifts occur only when light-induced Per gene expression spreads from the core to the shell SCN, with mPer1 expression in shell corresponding to phase advances, and mPer2 corresponding to phase delays. The results indicate that the time course and the localization of light-induced Per gene expression in SCN reveals important aspects of intra-SCN communication.
Figures






References
-
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. - PubMed
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of period gene in mice. Mol. Pharmacol. 2002;61:26–34. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mPer1 and mPer2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms. 2001;16:100–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

