Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer
- PMID: 12406730
- PMCID: PMC129931
- DOI: 10.1128/AEM.68.11.5394-5407.2002
Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer
Abstract
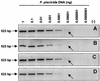
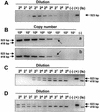
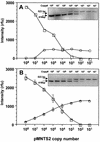
Pfiesteria piscicida is a heterotrophic dinoflagellate widely distributed along the middle Atlantic shore of the United States and associated with fish kills in the Neuse River (North Carolina) and the Chesapeake Bay (Maryland and Virginia). We constructed a genomic DNA library from clonally cultured P. piscicida and characterized the nontranscribed spacer (NTS), small subunit, internal transcribed spacer 1 (ITS1), 5.8S region, ITS2, and large subunit of the rRNA gene cluster. Based on the P. piscicida ribosomal DNA sequence, we developed a PCR-based detection assay that targets the NTS. The assay specificity was assessed by testing clonal P. piscicida and Pfiesteria shumwayae, 35 additional dinoflagellate species, and algal prey (Rhodomonas sp.). Only P. piscicida and nine presumptive P. piscicida isolates tested positive. All PCR-positive products yielded identical sequences for P. piscicida, suggesting that the PCR-based assay is species specific. The assay can detect a single P. piscicida zoospore in 1 ml of water, 10 resting cysts in 1 g of sediment, or 10 fg of P. piscicida DNA in 1 micro g of heterologous DNA. An internal standard for the PCR assay was constructed to identify potential false-negative results in testing of environmental sediment and water samples and as a competitor for the development of a quantitative competitive PCR assay format. The specificities of both qualitative and quantitative PCR assay formats were validated with >200 environmental samples, and the assays provide simple, rapid, and accurate methods for the assessment of P. piscicida in water and sediments.
Figures






References
-
- Allen, J. R., M. Roberts, A. R. Loeblich III, and L. C. Klotz. 1975. Characterization of the DNA from the dinoflagellate Crypthecodinium cohnii and implications for nuclear organization. Cell 6:161-169. - PubMed
-
- Anderson, D. M., and B. A. Keafer. 1985. Dinoflagellate cyst dynamics in coastal and estuarine waters, p. 219-224. In D. M. Anderson, A. W. White, and D. G. Baden (ed.), Toxic dinoflagellates. Elsevier, New York, N.Y.
-
- Ausubel, M. F., R. Brent, R. E. Kingston, D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Escherichia coli, plasmids, and bacteriophages, p. 1-38. In M. F. Ausubel, R. Brent, R. E. Kingston, D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Short protocols in molecular biology, 4th ed. John Wiley & Sons, Inc., Hoboken, N.J.
-
- Bena, G., M. F. Jubier, I. Olivieri, and B. Lejeune. 1998. Ribosomal external and internal transcribed spacers: combined use in the phylogenetic analysis of Medicago (Leguminosae). J. Mol. Evol. 46:299-306. - PubMed
-
- Bever, C. T., Jr., L. Grattan, and J. G. Morris. 1998. Neurologic symptoms following Pfiesteria exposure: case report and literature review. Md. Med. J. 47:120-123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

