Cooperation of hTERT, SV40 T antigen and oncogenic Ras in tumorigenesis: a cell transplantation model using bovine adrenocortical cells
- PMID: 12407443
- PMCID: PMC1503663
- DOI: 10.1038/sj.neo.7900262
Cooperation of hTERT, SV40 T antigen and oncogenic Ras in tumorigenesis: a cell transplantation model using bovine adrenocortical cells
Abstract
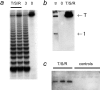
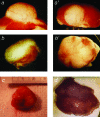
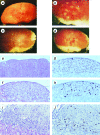
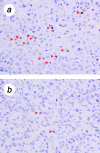
Expression of TERT, the reverse transcriptase component of telomerase, is necessary to convert normal human cells to cancer cells. Despite this, "telomerization" by hTERT does not appear to alter the normal properties of cells. In a cell transplantation model in which bovine adrenocortical cells form vascularized tissue structures beneath the kidney capsule in scid mice, telomerization does not perturb the functional tissue-forming capacity of the cells. This cell transplantation model was used to study the cooperation of hTERT with SV40 T antigen (SV40 TAg) and oncogenic Ras in tumorigenesis. Only cells expressing all three genes were tumorigenic; this required large T, but not small t, antigen. These cells produced a continuously expanding tissue mass; they were invasive with respect to adjacent organs and eventually destroyed the kidney. Cells expressing only hTERT or only Ras produced minimally altered tissues. In contrast, SV40 TAg alone produced noninvasive nodules beneath the kidney capsule that had high proliferation rates balanced by high rates of apoptosis. The use of cell transplantation techniques in a cell type that is able to form tissue structures with or without full neoplastic conversion allows the phenotypes produced by individual cooperating oncogenes to be observed.
Figures



References
-
- Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8:R178–R181. - PubMed
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
-
- Wright WE, Shay JW. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr Opin Genet Dev. 2001;11:98–103. - PubMed
-
- Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. - PubMed
-
- Thomas M, Yang L, Hornsby PJ. Formation of normal functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat Biotechnol. 2000;18:39–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
