Met-HGF/SF signal transduction induces mimp, a novel mitochondrial carrier homologue, which leads to mitochondrial depolarization
- PMID: 12407445
- PMCID: PMC1503665
- DOI: 10.1038/sj.neo.7900272
Met-HGF/SF signal transduction induces mimp, a novel mitochondrial carrier homologue, which leads to mitochondrial depolarization
Abstract
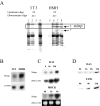
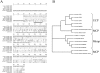
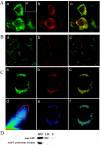
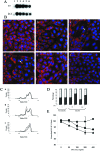
Met-hepatocyte growth factor/scatter factor (HGF/SF) signaling plays an important role in epithelial tissue morphogenesis, lumen formation, and tumorigenicity. We have recently demonstrated that HGF/SF also alters the metabolic activity of cells by enhancing both the glycolytic and oxidative phosphorylation pathways of energy production. Using differential display polymerase chain reaction, we cloned a novel gene, designated mimp (Met-Induced Mitochondrial Protein), which is upregulated in NIH-3T3 cells cotransfected with both HGF/SF and Met (HMH cells). Northern and Western blot analyses showed that mimp is induced in several Met-expressing cell lines following treatment with HGF/SF. Mimp encodes a 33-kDa protein that shows sequence homology to the family of mitochondrial carrier proteins (MCPs). Murine Mimp (mMimp) is expressed in a wide variety of tissues, exhibiting an expression pattern similar to Met. Predominant expression is seen in liver, kidney, heart, skeletal muscle, and testis. Using immunostaining for HA-tagged mMimp and a GFP-mMimp chimeric protein as well as subcellular fractionation, we determined that Mimp is primarily localized to the mitochondria. Ectopic expression of mMimp in the Met-responsive adenocarcinoma cell line, DA3, reduced the mitochondrial membrane potential (uncoupling activity). The extent of the mitochondrial depolarization positively correlated with the level of Mimp expression. Our results demonstrate that Mimp is a novel mitochondrial carrier homologue upregulated by Met-HGF/SF signal transduction, which leads to mitochondrial depolarization, and suggest novel links among tyrosine kinase signaling, mitochondrial function, and cellular bioenergetics.
Figures


References
-
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. - PubMed
-
- Bauer D, Warthoe P, Rohde M, Strauss M. Detection and differential display of expressed genes by DDRT-PCR. PCR Methods Appl. 1994;4:S97–S108. - PubMed
-
- Berdichevsky F, Alford D, D'Souza B, Taylor Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3557–3568. - PubMed
-
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
