Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes
- PMID: 12409481
- PMCID: PMC135848
- DOI: 10.1093/nar/gnf121
Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes
Abstract
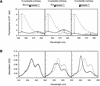
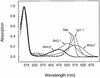
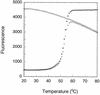
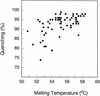
An important consideration in the design of oligonucleotide probes for homogeneous hybridization assays is the efficiency of energy transfer between the fluorophore and quencher used to label the probes. We have determined the efficiency of energy transfer for a large number of combinations of commonly used fluorophores and quenchers. We have also measured the quenching effect of nucleotides on the fluorescence of each fluorophore. Quenching efficiencies were measured for both the resonance energy transfer and the static modes of quenching. We found that, in addition to their photochemical characteristics, the tendency of the fluorophore and the quencher to bind to each other has a strong influence on quenching efficiency. The availability of these measurements should facilitate the design of oligonucleotide probes that contain interactive fluorophores and quenchers, including competitive hybridization probes, adjacent probes, TaqMan probes and molecular beacons.
Figures

References
-
- Heller M.J., Morrison,L.E., Prevatt,W.D. and Akin,C. (1983) Patent no. EPO070685.
-
- Morrison L.E., Halder,T.C. and Stols,L.M. (1989) Solution-phase detection of polynucleotides using interacting fluorescent labels and competitive hybridization. Anal. Biochem., 183, 231–244. - PubMed
-
- Livak K.J., Flood,S.J., Marmaro,J., Giusti,W. and Deetz,K. (1995) Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl., 4, 357–362. - PubMed
-
- Tyagi S. and Kramer,F.R. (1996) Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol., 14, 303–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

