Confirmation by FRET in individual living cells of the absence of significant amyloid beta -mediated caspase 8 activation
- PMID: 12409609
- PMCID: PMC137485
- DOI: 10.1073/pnas.232177599
Confirmation by FRET in individual living cells of the absence of significant amyloid beta -mediated caspase 8 activation
Abstract
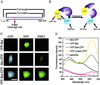
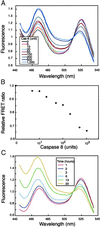
When cells are exposed to death-inducing molecules such as tumor necrosis factor-alpha or Fas, caspase 8 is activated and cleaves an apoptotic facilitator, Bid, that is a member of the Bcl-2 family. After additional modification, the C-terminal moiety of Bid is translocated to the mitochondria and induces the release of cytochrome c into the cytoplasm. In an attempt to directly observe the cleavage of Bid and the following events in living cells, we constructed a vector that encoded Bid fused with yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) (YFP-Bid-CFP). On expression of YFP-Bid-CFP in mammalian cells, we were able to observe the efficient transfer of energy from excited CFP to YFP within the YFP-Bid-CFP molecule and, importantly, the fusion protein YFP-Bid-CFP was fully functional in cells. When YFP-Bid-CFP was cleaved by caspase 8, on activation by anti-Fas Abs but not by Abeta or tunicamycin, no such transfer of energy was detected. To our knowledge, this is the first report of (i) visualization of the activation of Bid by proteolytic cleavage, with direct observation of the cleavage of YFP-Bid-CFP in the cytoplasm and subsequent translocation of the cleaved Bid to mitochondria and (ii) the absence of Abeta- or tunicamycin-mediated significant activation of caspase 8 in individual living cells.
Figures



References
-
- Li H. & Yuan, J. (1999) Curr. Opin. Cell Biol. 11, 261-266. - PubMed
-
- Korsmeyer S. J., Wei, M. C., Saito, M., Weiler, S., Oh, K. & Schlesinger, P. H. (2000) Cell Death Differ. 7, 1166-1173. - PubMed
-
- Wang K., Yin, X. M., Chao, D. T., Milliman, C. L. & Korsmeyer, S. J. (1996) Genes Dev. 10, 2859-2869. - PubMed
-
- Gross A., Yin, X. M., Wang, K., Wei, M. C., Jockel, J., Milliman, C., Erdjument-Bromage, H., Tempst, P. & Korsmeyer, S. J. (1999) J. Biol. Chem. 274, 1156-1163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

