Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme
- PMID: 12411478
- PMCID: PMC131063
- DOI: 10.1093/emboj/cdf566
Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme
Abstract
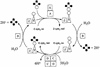
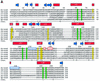
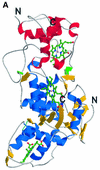
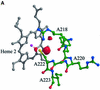
Reduced inorganic sulfur compounds are utilized by many bacteria as electron donors to photosynthetic or respiratory electron transport chains. This metabolism is a key component of the biogeochemical sulfur cycle. The SoxAX protein is a heterodimeric c-type cytochrome involved in thiosulfate oxidation. The crystal structures of SoxAX from the photosynthetic bacterium Rhodovulum sulfidophilum have been solved at 1.75 A resolution in the oxidized state and at 1.5 A resolution in the dithionite-reduced state, providing the first structural insights into the enzymatic oxidation of thiosulfate. The SoxAX active site contains a haem with unprecedented cysteine persulfide (cysteine sulfane) coordination. This unusual post-translational modification is also seen in sulfurtransferases such as rhodanese. Intriguingly, this enzyme shares further active site characteristics with SoxAX such as an adjacent conserved arginine residue and a strongly positive electrostatic potential. These similarities have allowed us to suggest a catalytic mechanism for enzymatic thiosulfate oxidation. The atomic coordinates and experimental structure factors have been deposited in the PDB with the accession codes 1H31, 1H32 and 1H33.
Figures






Similar articles
-
Novel heme ligation in a c-type cytochrome involved in thiosulfate oxidation: EPR and MCD of SoxAX from Rhodovulum sulfidophilum.Biochemistry. 2001 Sep 4;40(35):10562-9. doi: 10.1021/bi0100081. Biochemistry. 2001. PMID: 11523998
-
Novel domain packing in the crystal structure of a thiosulphate-oxidizing enzyme.Biochem Soc Trans. 2002 Aug;30(4):638-42. doi: 10.1042/bst0300638. Biochem Soc Trans. 2002. PMID: 12196153
-
Cytochrome complex essential for photosynthetic oxidation of both thiosulfate and sulfide in Rhodovulum sulfidophilum.J Bacteriol. 2001 Oct;183(20):6107-18. doi: 10.1128/JB.183.20.6107-6118.2001. J Bacteriol. 2001. PMID: 11567011 Free PMC article.
-
The bacterial SoxAX cytochromes.Cell Mol Life Sci. 2013 Mar;70(6):977-92. doi: 10.1007/s00018-012-1098-y. Epub 2012 Aug 21. Cell Mol Life Sci. 2013. PMID: 22907414 Free PMC article. Review.
-
C-type cytochromes in the photosynthetic electron transfer pathways in green sulfur bacteria and heliobacteria.Photosynth Res. 2010 Jun;104(2-3):189-99. doi: 10.1007/s11120-009-9521-4. Epub 2010 Jan 21. Photosynth Res. 2010. PMID: 20091230 Review.
Cited by
-
Mechanism of thiosulfate oxidation in the SoxA family of cysteine-ligated cytochromes.J Biol Chem. 2015 Apr 3;290(14):9209-21. doi: 10.1074/jbc.M114.618025. Epub 2015 Feb 11. J Biol Chem. 2015. PMID: 25673696 Free PMC article.
-
Electron Accepting Units of the Diheme Cytochrome c TsdA, a Bifunctional Thiosulfate Dehydrogenase/Tetrathionate Reductase.J Biol Chem. 2016 Nov 25;291(48):24804-24818. doi: 10.1074/jbc.M116.753863. Epub 2016 Sep 30. J Biol Chem. 2016. PMID: 27694441 Free PMC article.
-
Genomes of Neutrophilic Sulfur-Oxidizing Chemolithoautotrophs Representing 9 Proteobacterial Species From 8 Genera.Front Microbiol. 2019 Feb 25;10:316. doi: 10.3389/fmicb.2019.00316. eCollection 2019. Front Microbiol. 2019. PMID: 30858836 Free PMC article.
-
The antioxidant and oxidant properties of hydropersulfides (RSSH) and polysulfide species.Redox Biol. 2022 Nov;57:102486. doi: 10.1016/j.redox.2022.102486. Epub 2022 Sep 25. Redox Biol. 2022. PMID: 36201912 Free PMC article.
-
Properties and structure of a low-potential, penta-heme cytochrome c552 from a thermophilic purple sulfur photosynthetic bacterium Thermochromatium tepidum.Photosynth Res. 2019 Mar;139(1-3):281-293. doi: 10.1007/s11120-018-0507-y. Epub 2018 Apr 24. Photosynth Res. 2019. PMID: 29691716
References
-
- Bartsch R.G. (1971) Cytochromes: bacterial. Methods Enzymol., 23, 344–363.
-
- Beinert H. (1983) Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron–sulfur proteins. Anal. Biochem., 131, 373–378. - PubMed
-
- Bordo D., Deriu,D., Colnaghi,R., Carpen,A., Pagani,S. and Bolognesi,M. (2000) The crystal structure of a sulfurtransferase from Azotobacter vinelandii highlights the evolutionary relationship between the rhodanese and phosphatase enzyme families. J. Mol. Biol., 298, 691–704. - PubMed
-
- Brune D.C. (1989) Sulfur oxidation by phototrophic bacteria. Biochim. Biophys. Acta, 975, 189–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

