Reversible stages of the low-pH-triggered conformational change in influenza virus hemagglutinin
- PMID: 12411488
- PMCID: PMC131056
- DOI: 10.1093/emboj/cdf559
Reversible stages of the low-pH-triggered conformational change in influenza virus hemagglutinin
Abstract
The refolding of the prototypic fusogenic protein hemagglutinin (HA) at the pH of fusion is considered to be a concerted and irreversible discharge of a loaded spring, with no distinct intermediates between the initial and final conformations. Here, we show that HA refolding involves reversible conformations with a lifetime of minutes. After reneutralization, low pH-activated HA returns from the conformations wherein both the fusion peptide and the kinked loop of the HA2 subunit are exposed, but the HA1 subunits have not yet dissociated, to a structure indistinguishable from the initial one in functional, biochemical and immunological characteristics. The rate of the transition from reversible conformations to irreversible refolding depends on the pH and on the presence of target membrane. Importantly, recovery of the initial conformation is blocked by the interactions between adjacent HA trimers. The existence of the identified reversible stage of refolding can be crucial for allowing multiple copies of HA to synchronize their release of conformational energy, as required for fusion.
Figures
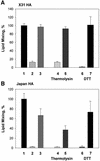
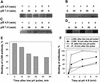

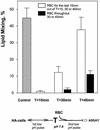
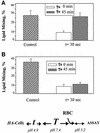
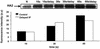
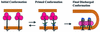
References
-
- Bullough P.A., Hughson,F.M., Skehel,J.J. and Wiley,D.C. (1994) Structure of influenza haemagglutinin at the pH of membrane fusion. Nature, 371, 37–43. - PubMed
-
- Carr C.M. and Kim,P.S. (1993) A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell, 73, 823–832. - PubMed
-
- Chen J., Lee,K.H., Steinhauer,D.A., Stevens,D.J., Skehel,J.J. and Wiley,D.C. (1998) Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell, 95, 409–417. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

