Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA
- PMID: 12411491
- PMCID: PMC131072
- DOI: 10.1093/emboj/cdf575
Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA
Abstract
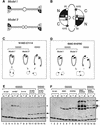
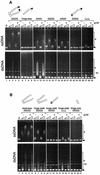
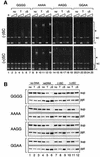
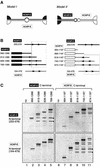
Structural maintenance of chromosomes (SMC) proteins play central roles in regulating higher order chromosome dynamics from bacteria to humans. As judged by electron microscopy, the SMC homodimer from Bacillus subtilis (BsSMC) is composed of two antiparallel, coiled-coil arms with a flexible hinge. Site-directed cross-linking experiments show here that dimerization of BsSMC is mediated by a hinge-hinge interaction between self-folded monomers. This architecture is conserved in the eukaryotic SMC2-SMC4 heterodimer. Analysis of different deletion mutants of BsSMC unexpectedly reveals that the major DNA-binding activity does not reside in the catalytic ATPase domains located at the ends of a dimer. Instead, point mutations in the hinge domain that disturb dimerization of BsSMC drastically reduce its ability to interact with DNA. Proper hinge function is essential for BsSMC to recognize distinct DNA topology, and mutant proteins with altered hinge angles cross-link double-stranded DNA in a nucleotide-dependent manner. We propose that the hinge domain of SMC proteins is not a simple dimerization site, but rather it acts as an essential determinant of dynamic SMC-DNA interactions.
Figures



References
-
- Akhmedov A.T., Frei,C., Tsai-Pflugfelder,M., Kemper,B., Gasser,S.M. and Jessberger,R. (1998) Structural maintenance of chromosomes protein C-terminal domains bind preferentially to DNA with secondary structure. J. Biol. Chem., 273, 24088–24094. - PubMed
-
- Akhmedov A.T., Gross,B. and Jessberger,R. (1999) Mammalian SMC3 C-terminal and coiled-coil protein domains specifically bind palindromic DNA, do not block DNA ends and prevent DNA bending. J. Biol. Chem., 274, 38216–38224. - PubMed
-
- Bazett-Jones D.P., Kimura,K. and Hirano,T. (2002) Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol. Cell, 9, 1183–1190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

