Importin alpha can migrate into the nucleus in an importin beta- and Ran-independent manner
- PMID: 12411501
- PMCID: PMC131066
- DOI: 10.1093/emboj/cdf569
Importin alpha can migrate into the nucleus in an importin beta- and Ran-independent manner
Abstract
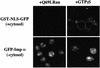
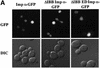
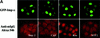
A classical nuclear localization signal (NLS)-containing protein is transported into the nucleus via the formation of a NLS-substrate/importin alpha/beta complex. In this study, we found that importin alpha migrated into the nucleus without the addition of importin beta, Ran or any other soluble factors in an in vitro transport assay. A mutant importin alpha lacking the importin beta-binding domain efficiently entered the nucleus. Competition experiments showed that this import pathway for importin alpha is distinct from that of importin beta. These results indicate that importin alpha alone can enter the nucleus via a novel pathway in an importin beta- and Ran-independent manner. Furthermore, this process is evolutionarily conserved as similar results were obtained in Saccharomyces cerevisiae. Moreover, the import rate of importin alpha differed among individual nuclei of permeabilized cells, as demonstrated by time-lapse experiments. This heterogeneous nuclear accumulation of importin alpha was affected by the addition of ATP, but not ATPgammaS. These results suggest that the nuclear import machinery for importin alpha at individual nuclear pore complexes may be regulated by reaction(s) that require ATP hydrolysis.
Figures











Similar articles
-
Characterization of nuclear import of the domain-specific androgen receptor in association with the importin alpha/beta and Ran-guanosine 5'-triphosphate systems.Endocrinology. 2008 Aug;149(8):3960-9. doi: 10.1210/en.2008-0137. Epub 2008 Apr 17. Endocrinology. 2008. PMID: 18420738 Free PMC article.
-
The importin-beta binding domain of snurportin1 is responsible for the Ran- and energy-independent nuclear import of spliceosomal U snRNPs in vitro.J Cell Biol. 2002 Feb 4;156(3):467-79. doi: 10.1083/jcb.200108114. Epub 2002 Jan 28. J Cell Biol. 2002. PMID: 11815630 Free PMC article.
-
Influence of cargo size on Ran and energy requirements for nuclear protein import.J Cell Biol. 2002 Oct 14;159(1):55-67. doi: 10.1083/jcb.200204163. Epub 2002 Oct 7. J Cell Biol. 2002. PMID: 12370244 Free PMC article.
-
Nucleocytoplasmic protein transport and recycling of Ran.Cell Struct Funct. 1999 Dec;24(6):425-33. doi: 10.1247/csf.24.425. Cell Struct Funct. 1999. PMID: 10698256 Review.
-
Molecular mechanisms of nuclear protein transport.Crit Rev Eukaryot Gene Expr. 1997;7(1-2):61-72. doi: 10.1615/critreveukargeneexpr.v7.i1-2.40. Crit Rev Eukaryot Gene Expr. 1997. PMID: 9034715 Review.
Cited by
-
Diversification of importin-α isoforms in cellular trafficking and disease states.Biochem J. 2015 Feb 15;466(1):13-28. doi: 10.1042/BJ20141186. Biochem J. 2015. PMID: 25656054 Free PMC article. Review.
-
Drosophila importin alpha1 performs paralog-specific functions essential for gametogenesis.Genetics. 2008 Feb;178(2):839-50. doi: 10.1534/genetics.107.081778. Epub 2008 Feb 1. Genetics. 2008. PMID: 18245351 Free PMC article.
-
Pressurized DNA state inside herpes capsids-A novel antiviral target.PLoS Pathog. 2020 Jul 23;16(7):e1008604. doi: 10.1371/journal.ppat.1008604. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32702029 Free PMC article.
-
Probing nuclear localization signal-importin alpha binding equilibria in living cells.J Biol Chem. 2009 Dec 25;284(52):36638-36646. doi: 10.1074/jbc.M109.036699. Epub 2009 Oct 26. J Biol Chem. 2009. PMID: 19858191 Free PMC article.
-
Landscape of nuclear transport receptor cargo specificity.Mol Syst Biol. 2017 Dec 18;13(12):962. doi: 10.15252/msb.20177608. Mol Syst Biol. 2017. PMID: 29254951 Free PMC article.
References
-
- Bischoff F.R. and Ponstingl,H. (1991) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature, 354, 80–82. - PubMed
-
- Booth J.W., Belanger,K.D., Sannella,M.I. and Davis,L.I. (1999) The yeast nucleoporin Nup2p is involved in nuclear export of importin α/Srp1p. J. Biol. Chem., 274, 32360–32367. - PubMed
-
- Conti E., Uy,M., Leighton,L., Blobel,G. and Kuriyan,J. (1998) Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin α. Cell, 94, 193–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

