Contributions of the non-alpha subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor
- PMID: 12411516
- PMCID: PMC2290637
- DOI: 10.1113/jphysiol.2002.029413
Contributions of the non-alpha subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor
Abstract
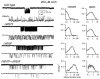
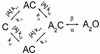
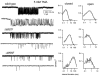
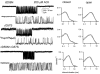
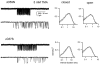
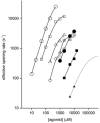
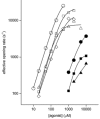
The agonist binding site of the nicotinic acetylcholine receptor has a loop-based structure, and is formed by residues located remotely to each other in terms of primary structure. Amino acid residues in sites delta57 and delta59, and the equivalent residues in the epsilon; subunit, have been identified as part of the agonist binding site and designated as loop D. The effects of point mutations in sites delta57, delta59, epsilon;55 and epsilon;57 on agonist binding and channel gating were studied. The mutated receptors were expressed transiently in HEK 293 cells and the currents were recorded using the cell-attached single-channel patch clamp technique. The results demonstrate that the mutations mainly affect channel gating with the major portion of the effect due to a reduction in the channel opening rate constant. For both the delta57/epsilon;55 and the delta59/epsilon;57 site, a mutation in the epsilon; subunit had a greater effect on channel gating than a mutation in the delta subunit. In all instances, agonist binding was affected to a lesser degree than channel gating. Previous data have placed the delta57 and delta59 residues in or near the agonist binding pocket. The data presented here suggest that these two residues (and the homologous sites in the epsilon; subunit) are not involved in specific interactions with the nicotinic agonist and that they affect the activation of the nicotinic receptor by shaping the overall structure of the agonist binding site.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

