Fast synaptic transmission mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in lamina X neurones of neonatal rat spinal cord
- PMID: 12411519
- PMCID: PMC2290641
- DOI: 10.1113/jphysiol.2002.028894
Fast synaptic transmission mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in lamina X neurones of neonatal rat spinal cord
Abstract
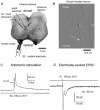
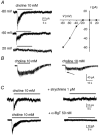
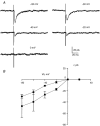
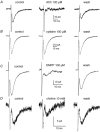
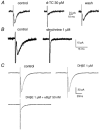
Using patch clamp recordings on neonatal rat spinal cord slices, we have looked for the presence of alpha-bungarotoxin-sensitive nicotinic ACh receptors (nAChRs) on sympathetic preganglionic neurones (SPNs) surrounding the central canal of the spinal cord (lamina X) and examined whether they were implicated in a fast cholinergic synaptic transmission. SPNs were identified either by their morphology using biocytin in the recording electrode and/or by antidromic stimulation of the ventral rootlets. The selective alpha7-containing nAChR (alpha7*nAChR) agonist choline (10 mM) induced a fast, rapidly desensitizing inward current, which was fully blocked by alpha-bungarotoxin (alpha-BgT; 50 nM) and strychnine (1 microM), two antagonists of alpha7*nAChRs. The I-V relationship of the choline-induced current showed a strong inward-going rectification. Electrically evoked excitatory postsynaptic currents (eEPSCs) could be recorded. At -60 mV, eEPSCs peaked at -26.2 pA and decayed monoexponentially with a mean time constant of 8.5 ms. The current-voltage relationship for eEPSCs exhibited a strong inward rectification and a reversal potential close to 0 mV, compatible with a non-selective cationic current. The appearance of eEPSCs was entirely suppressed by the application of 100 microM ACh or nicotine. Choline (10 mM) and 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP; 100 microM) both reduced the amplitude of eEPSCs, whereas cytisine (100 microM) had no effect. Strychnine (1 microM) and alpha-BgT (50 nM) both suppressed the eEPSCs. Blocking the P2X purinergic and 5-HT(3) receptors had no effect on eEPSCs. DMPP induced four types of current, which differed in their onset and desensitization rate. The most frequently encountered responses were insensitive to the action of strychnine and alpha-BgT, and were reproduced by ACh and nicotine but not by cytisine. We conclude that SPNs of the lamina X express several classes of nAChRs and in particular alpha-BgT-sensitive nAChRs. This is the first demonstration in a mammalian spinal cord preparation of a fast cholinergic neurotransmission in which alpha-BgT-sensitive nicotinic receptors are involved.
Figures


References
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons: I Pharmacological and functional evidence for distinct structural subtypes. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1455–1473. - PubMed
-
- Alkondon M, Pereira EF, Albuquerque EX. Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Research. 1998;810:257–263. - PubMed
-
- Arias HR. Agonist self-inhibitory binding site of the nicotinic acetylcholine receptor. Journal of Neuroscience Research. 1996;44:97–105. - PubMed
-
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing acetyltransferase in the adult rat spinal cord: an immunocytochemical study. Journal of Comparative Neurology. 1984;229:329–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

