Recruitment of Ca(2+) release channels by calcium-induced Ca(2+) release does not appear to occur in isolated Ca(2+) release sites in frog skeletal muscle
- PMID: 12411523
- PMCID: PMC2290617
- DOI: 10.1113/jphysiol.2002.026658
Recruitment of Ca(2+) release channels by calcium-induced Ca(2+) release does not appear to occur in isolated Ca(2+) release sites in frog skeletal muscle
Abstract
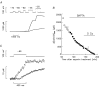
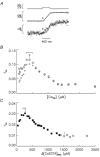
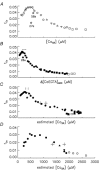
Ca(2+) release from the sarcoplasmic reticulum (SR) in skeletal muscle in response to small depolarisations (e.g. to -60 mV) should be the sum of release from many isolated Ca(2+) release sites. Each site has one SR Ca(2+) release channel activated by its associated T-tubular voltage sensor. The aim of this study was to evaluate whether it also includes neighbouring Ca(2+) release channels activated by Ca-induced Ca(2+) release (CICR). Ca(2+) release in frog cut muscle fibres was estimated with the EGTA/phenol red method. The fraction of SR Ca content ([Ca(SR)]) released by a 400 ms pulse to -60 mV (denoted f(Ca)) provided a measure of the average Ca(2+) permeability of the SR associated with the pulse. In control experiments, f(Ca) was approximately constant when [Ca(SR)] was 1500-3000 microM (plateau region) and then increased as [Ca(SR)] decreased, reaching a peak when [Ca(SR)] was 300-500 microM that was 4.8 times larger on average than the plateau value. With 8 mM of the fast Ca(2+) buffer BAPTA in the internal solution, f(Ca) was 5.0-5.3 times larger on average than the plateau value obtained before adding BAPTA when [Ca(SR)] was 300-500 microM. In support of earlier results, 8 mM BAPTA did not affect Ca(2+) release in the plateau region. At intermediate values of [Ca(SR)], BAPTA resulted in a small, if any, increase in f(Ca), presumably by decreasing Ca inactivation of Ca(2+) release. Since BAPTA never decreased f(Ca), the results indicate that neighbouring channels are not activated by CICR with small depolarisations when [Ca(SR)] is 300-3000 microM.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

