The structure and evolution of the major capsid protein of a large, lipid-containing DNA virus
- PMID: 12411581
- PMCID: PMC137492
- DOI: 10.1073/pnas.232580699
The structure and evolution of the major capsid protein of a large, lipid-containing DNA virus
Abstract
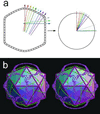
Paramecium bursaria Chlorella virus type 1 (PBCV-1) is a very large, icosahedral virus containing an internal membrane enclosed within a glycoprotein coat consisting of pseudohexagonal arrays of trimeric capsomers. Each capsomer is composed of three molecules of the major capsid protein, Vp54, the 2.0-A resolution structure of which is reported here. Four N-linked and two O-linked glycosylation sites were identified. The N-linked sites are associated with nonstandard amino acid motifs as a result of glycosylation by virus-encoded enzymes. Each monomer of the trimeric structure consists of two eight-stranded, antiparallel beta-barrel, "jelly-roll" domains related by a pseudo-sixfold rotation. The fold of the monomer and the pseudo-sixfold symmetry of the capsomer resembles that of the major coat proteins in the double-stranded DNA bacteriophage PRD1 and the double-stranded DNA human adenoviruses, as well as the viral proteins VP2-VP3 of picornaviruses. The structural similarities among these diverse groups of viruses, whose hosts include bacteria, unicellular eukaryotes, plants, and mammals, make it probable that their capsid proteins have evolved from a common ancestor that had already acquired a pseudo-sixfold organization. The trimeric capsid protein structure was used to produce a quasi-atomic model of the 1,900-A diameter PBCV-1 outer shell, based on fitting of the Vp54 crystal structure into a three-dimensional cryoelectron microscopy image reconstruction of the virus.
Figures



References
-
- Tidona C. A., Schnitzler, P., Kehm, R. & Darai, G. (1998) Virus Genes 16, 59-66. - PubMed
-
- Dixon L. K., Rock, D. & Vinuela, E. (1995) in Virus Taxonomy, eds. Murphy, F. A., Fauquet, C. M., Bishop, D. H. L., Ghabrial, S. A., Jarvis, A. W., Martelli, G. P., Mayo, M. A. & Summers, M. D. (Springer, New York), pp. 92–94.
-
- Meints R. H., Lee, K., Burbank, D. E. & Van Etten, J. L. (1984) Virology 138, 341-346. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

