A role for caspase-1 and -3 in the pathology of experimental allergic encephalomyelitis : inflammation versus degeneration
- PMID: 12414506
- PMCID: PMC1850770
- DOI: 10.1016/S0002-9440(10)64436-7
A role for caspase-1 and -3 in the pathology of experimental allergic encephalomyelitis : inflammation versus degeneration
Abstract
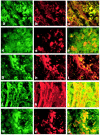
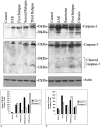
Axonal loss, already present in the acute and first relapse phases of experimental allergic encephalomyelitis (EAE) in the ABH mouse, only becomes apparent in the third relapse in the interleukin-12 model of relapsing EAE in the Lewis rat. Caspase-1 immunostaining in the spinal cord of Lewis rats was mainly localized to inflammatory cuffs with the greatest proportion of active caspase-1-positive cells detected during the first and second relapses, correlating with enzyme activity and protein on Western blots. However, in the spinal cord of ABH mice during acute EAE, caspase-1 immunostaining was localized both on inflammatory and neuronal cells, again correlating with enzyme activity and protein production. In contrast, caspase-3 expression in the spinal cord of Lewis rats did not increase significantly until the third relapse when inflammatory and neuronal cells and axons became positive in line with a significant increase in caspase activity. In ABH mice active caspase-3 was already immunolocalized on axons and apoptotic neurons in the spinal cord during the acute stage of EAE. Because caspase-3 is a downstream cell death signal it may be possible to reduce apoptosis by selectively blocking caspase-3 and therefore provide a therapeutic target for EAE and potentially, multiple sclerosis.
Figures




References
-
- Baker D, O’Neill JK, Gschmeissner SE, Wilcox CE, Butter C, Turk JL: Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice. J Neuroimmunol 1990, 28:261-270 - PubMed
-
- Thornberry NA, Lazebnik Y: Caspases: enemies within. Science 1998, 281:1312-1316 - PubMed
-
- Arends MJ, Wyllie AH: Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol 1991, 32:223-254 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

