Proliferation, but not apoptosis, is associated with distinct beta-catenin expression patterns in non-small-cell lung carcinomas: relationship with adenomatous polyposis coli and G(1)-to S-phase cell-cycle regulators
- PMID: 12414510
- PMCID: PMC1850775
- DOI: 10.1016/s0002-9440(10)64440-9
Proliferation, but not apoptosis, is associated with distinct beta-catenin expression patterns in non-small-cell lung carcinomas: relationship with adenomatous polyposis coli and G(1)-to S-phase cell-cycle regulators
Abstract
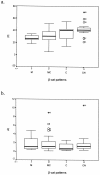
beta-catenin (beta-cat) is a versatile component of homotypic cell adhesion and signaling. Its subcellular localization and cytoplasmic levels are tightly regulated by the adenomatous polyposis coli (APC) protein. Mutations in beta-cat (exon 3) or APC (MCR) result in beta-cat aberrant overexpression that is associated with its nuclear accumulation and improper gene activation. Data from experimental models have shown that beta-cat overexpression has a multitude of effects on cell-cycle behavior. In many of these aspects its function depends on major G(1) phase regulators. To the best of our knowledge, most of these issues have never been addressed concurrently in tumors. For this reason we investigated in a panel of 92 non-small-cell lung carcinomas, beta-cat and APC expression, and their relationship with cell-cycle kinetics (PI and AI) and ploidy status. Moreover, the above correlations were examined in relation to the main G(1)/S-phase checkpoint regulators. Four beta-cat immunohistochemical expression patterns [membranous (11.1%), membranous-cytoplasmic (54.3%), cytoplasmic (9.9%), cytoplasmic-nuclear (24.7%)] and three APC immunohistochemical expression patterns [cytoplasmic (37.7%), cytoplasmic-nuclear (58%), nuclear (4.3%)] were observed, which were further confirmed by Western blot analysis on subcellular fractions in representative samples. The frequent presence of beta-cat in the cytoplasm is an indication of aberrant expression, whereas membranous and nuclear localization were inversely related. Absence of mutations in beta-cat (exon 3) and APC (MCR) suggest that beta-cat destruction mechanisms may be functional. However, expression analysis revealed attenuated levels for APC, indicating a residual ability to degrade beta-cat. Decreased levels were associated with loss of heterozygosity at the APC region in 24% of the cases suggesting that additional silencing mechanisms may be involved. Interestingly, the 90-kd APC isoform associated with apoptosis, was found to be the predominant isoform in normal and cancerous lung tissues. The most important finding in our study, was the correlation of nuclear beta-cat immunohistochemical localization with increased proliferation, overexpression of E2F1 and MDM2, aberrant p53, and low expression of p27(KIP), providing for the first time in vivo evidence that beta-cat-associated proliferation correlates with release of E2F1 activity and loss of p53- and p27(KIP)-dependent cell-cycle checkpoints. Loss of these checkpoints is accompanied by low levels of APC, which possibly reflects a diminished ability to degrade beta-cat. Taken together our data indicate that cases with nuclear beta-cat immunohistochemical expression represent a subset of non-small-cell lung carcinomas that have gained an increased proliferation advantage in contrast to the other beta-cat immunohistochemical expression profiles.
Figures


Similar articles
-
Suppression of kinesin expression disrupts adenomatous polyposis coli (APC) localization and affects beta-catenin turnover in young adult mouse colon (YAMC) epithelial cells.Exp Cell Res. 2002 Oct 15;280(1):12-23. doi: 10.1006/excr.2002.5506. Exp Cell Res. 2002. PMID: 12372335
-
Abnormalities of the APC/beta-catenin pathway in endometrial cancer.Oncogene. 2002 Nov 14;21(52):7981-90. doi: 10.1038/sj.onc.1205924. Oncogene. 2002. PMID: 12439748
-
Nuclear accumulation of full-length and truncated adenomatous polyposis coli protein in tumor cells depends on proliferation.Oncogene. 2003 Sep 4;22(38):6013-22. doi: 10.1038/sj.onc.1206731. Oncogene. 2003. PMID: 12955080
-
Nuclear accumulation of beta-catenin without an additional somatic mutation in coding region of the APC gene in hepatoblastoma from a familial adenomatous polyposis patient.Oncol Rep. 2004 Jan;11(1):121-6. Oncol Rep. 2004. PMID: 14654913 Review.
-
The ins and outs of APC and beta-catenin nuclear transport.EMBO Rep. 2002 Sep;3(9):834-9. doi: 10.1093/embo-reports/kvf181. EMBO Rep. 2002. PMID: 12223464 Free PMC article. Review.
Cited by
-
Wnt pathway activation predicts increased risk of tumor recurrence in patients with stage I nonsmall cell lung cancer.Ann Surg. 2013 Mar;257(3):548-54. doi: 10.1097/SLA.0b013e31826d81fd. Ann Surg. 2013. PMID: 23011390 Free PMC article.
-
WNT signaling - lung cancer is no exception.Respir Res. 2017 Sep 5;18(1):167. doi: 10.1186/s12931-017-0650-6. Respir Res. 2017. PMID: 28870231 Free PMC article. Review.
-
Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability--evidence of E2F-1 transcriptional control over hCdt1.Am J Pathol. 2004 Oct;165(4):1351-65. doi: 10.1016/S0002-9440(10)63393-7. Am J Pathol. 2004. PMID: 15466399 Free PMC article.
-
Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations.Clin Exp Med. 2011 Jun;11(2):105-12. doi: 10.1007/s10238-010-0110-9. Epub 2010 Aug 31. Clin Exp Med. 2011. PMID: 20809334
-
Wnt signalling in lung development and diseases.Respir Res. 2006 Jan 26;7(1):15. doi: 10.1186/1465-9921-7-15. Respir Res. 2006. PMID: 16438732 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000, 100:57-70 - PubMed
-
- Peifer M, Polakis P: Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000, 287:1606-1609 - PubMed
-
- Ilyas M, Tomlinson IPM: The interactions of APC, E-cadherin and β-catenin in tumor development and progression. J Pathol 1997, 182:128-137 - PubMed
-
- Morin PJ: β-catenin signaling and cancer. BioEssays 1999, 21:1021-1030 - PubMed
-
- Barker N, Clevers H: Catenins, Wnt signaling and cancer. BioEssays 2000, 22:961-965 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

