Immunosuppressive treatment protects against angiotensin II-induced renal damage
- PMID: 12414515
- PMCID: PMC1850776
- DOI: 10.1016/S0002-9440(10)64445-8
Immunosuppressive treatment protects against angiotensin II-induced renal damage
Abstract
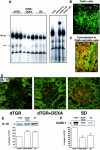
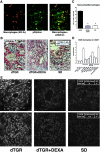
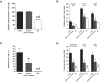
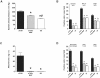
Angiotensin (Ang) II promotes renal infiltration by immunocompetent cells in double-transgenic rats (dTGRs) harboring both human renin and angiotensinogen genes. To elucidate disease mechanisms, we investigated whether or not dexamethasone (DEXA) immunosuppression ameliorates renal damage. Untreated dTGRs developed hypertension, renal damage, and 50% mortality at 7 weeks. DEXA reduced albuminuria, renal fibrosis, vascular reactive oxygen stress, and prevented mortality, independent of blood pressure. In dTGR kidneys, p22phox immunostaining co-localized with macrophages and partially with T cells. dTGR dendritic cells expressed major histocompatibility complex II and CD86, indicating maturation. DEXA suppressed major histocompatibility complex II+, CD86+, dendritic, and T-cell infiltration. In additional experiments, we treated dTGRs with mycophenolate mofetil to inhibit T- and B-cell proliferation. Reno-protective actions of mycophenolate mofetil and its effect on dendritic and T cells were similar to those obtained with DEXA. We next investigated whether or not Ang II directly promotes dendritic cell maturation in vitro. Ang II did not alter CD80, CD83, and MHC II expression, but increased CCR7 expression and cell migration. To explore the role of tumor necrosis factor (TNF)-alpha on dendritic cell maturation in vivo, we treated dTGRs with the soluble TNF-alpha receptor etanercept. This treatment had no effect on blood pressure, but decreased albuminuria, nuclear factor-kappaB activation, and infiltration of all immunocompetent cells. These data suggest that immunosuppression prevents dendritic cell maturation and T-cell infiltration in a nonimmune model of Ang II-induced renal damage. Ang II induces dendritic migration directly, whereas in vivo TNF-alpha is involved in dendritic cell infiltration and maturation. Thus, Ang II may initiate events leading to innate and acquired immune response.
Figures




References
-
- Rodriguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ: Role of immunocompetent cells in nonimmune renal diseases. Kidney Int 2001, 59:1626-1640 - PubMed
-
- Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ: Salt-sensitive hypertension develops after short-term exposure to Angiotensin II. Hypertension 1999, 33:1013-1019 - PubMed
-
- Mai M, Geiger H, Hilgers KF, Veelken R, Mann JF, Dammrich J, Luft FC: Early interstitial changes in hypertension-induced renal injury. Hypertension 1993, 22:754-765 - PubMed
-
- Grafe M, Auch-Schwelk W, Zakrzewicz A, Regitz-Zagrosek V, Bartsch P, Graf K, Loebe M, Gaehtgens P, Fleck E: Angiotensin II-induced leukocyte adhesion on human coronary endothelial cells is mediated by E-selectin. Circ Res 1997, 81:804-811 - PubMed
-
- Krejcy K, Eichler HG, Jilma B, Kapiotis S, Wolzt M, Zanaschka G, Gasic S, Schutz W, Wagner O: Influence of angiotensin II on circulating adhesion molecules and blood leukocyte count in vivo. Can J Physiol Pharmacol 1996, 74:9-14 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

