Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms
- PMID: 12414517
- PMCID: PMC1850780
- DOI: 10.1016/S0002-9440(10)64447-1
Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms
Abstract
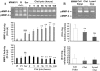
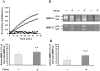
Acquired abdominal aortic aneurysms are usually associated with a mural thrombus through which blood continues to flow. Some early data suggest that aneurysmal evolution correlates with the biological activity of the thrombus. Our hypothesis was therefore that the thrombus could adsorb blood components and store, release, and participate in the activation of proteases involved in aneurysmal evolution. For this purpose, we have explored both the metalloproteinase and fibrinolytic systems in the thrombus and the wall of human aneurysms. We have first investigated blood clot formation and lysis in vitro. Spontaneous clotting induces a release of promatrix metalloproteinase (pro-MMP)-9 into the serum that was fourfold higher than in paired control plasma (P < 0.001). Fibrinolysis progressively released more MMP-9 in a time-dependent manner (P < 0.01). After selective isolation, we demonstrated that polymorphonuclear leukocytes are the main source of MMP-9 release during clot formation. Protease content was then analyzed in 35 mural thrombi and walls of human abdominal aortic aneurysms sampled during surgical repair. In 15 aneurysms, the liquid phase at the interface between the thrombus and the wall was sampled separately. Both thrombus and wall contained MMP-2 and MMP-9 but the ratio MMP-9/MMP-2 was higher in the thrombus than in the wall. The liquid interface also contained active MMP-9. Immunohistochemistry of the thrombus confirmed these findings, showing the presence of polymorphonuclear leukocytes at the luminal pole of the thrombus, co-localizing with MMP-9 storage. In contrast, MMP-3 and MMP-7 were only present in the aneurysmal wall. Plasminogen was present in the mural thrombus but plasmin activity was present in both thrombus and wall. In the liquid interface, plasmin-alpha(2)-anti-plasmin complexes were detected demonstrating in vivo the activation of plasminogen. In contrast, u-PA and t-PA were detectable only in the wall, suggesting that plasminogen present in the thrombus could be activated by factors secreted by the arterial wall. This was demonstrated in vitro, in which co-incubation of thrombus and wall extracts generated plasmin in the presence of a fibrin matrix and activated MMPs. In conclusion, our study strongly suggests that the mural thrombus, by trapping polymorphonuclear leukocytes and adsorbing plasma components could act as a source of proteases in aneurysms that may play a critical role in enlargement and rupture.
Figures





References
-
- Michel JB: Contrasting outcomes of atheroma evolution: intimal accumulation versus medial destruction. Arterioscler Thromb Vasc Biol 2001, 21:1389-1392 - PubMed
-
- Busutill R, Rinderbreich H, Flesher A, Camarck C: Elastase activity, the role of elastase in aortic aneurysm formation. J Surg Res 1982, 32:214-217 - PubMed
-
- Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB: Elastase-induced experimental aneurysms in rats. Circulation 1990, 82:973-981 - PubMed
-
- Shireman P, McCarthy W, Pearce W, Shively V, Cipollone M, Kwaan H: Elevation of tissue-type plasminogen activator and differential expression of urokinase-type plasminogen activator in diseased aorta. J Vasc Surg 1997, 25:157-164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

