Capture and transfer of simian immunodeficiency virus by macaque dendritic cells is enhanced by DC-SIGN
- PMID: 12414925
- PMCID: PMC136877
- DOI: 10.1128/jvi.76.23.11827-11836.2002
Capture and transfer of simian immunodeficiency virus by macaque dendritic cells is enhanced by DC-SIGN
Abstract
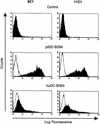
Dendritic cells (DCs) are among the first cells encountered by human and simian immunodeficiency virus (HIV and SIV) following mucosal infection. Because these cells efficiently capture and transmit virus to T cells, they may play a major role in mediating HIV and SIV infection. Recently, a C-type lectin protein present on DCs, DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), was shown to efficiently bind and present HIV and SIV to CD4(+), coreceptor-positive cells in trans. However, the significance of DC-SIGN for virus transmission and pathogenesis in vivo remains unclear. Because SIV infection of macaques may represent the best model to study the importance of DC-SIGN in HIV infection, we cloned and characterized pig-tailed macaque DC-SIGN and generated monoclonal antibodies (MAbs) against it. We demonstrate that, like human DC-SIGN, pig-tailed macaque DC-SIGN (ptDC-SIGN) is expressed on DCs and macrophages but not on monocytes, T cells, or B cells. Moderate levels of ptDC-SIGN expression were detected on the surface of DCs, and low-level expression was found on macrophages. Additionally, we show that ptDC-SIGN efficiently binds and transmits replication-competent SIVmne variants to CD4(+), coreceptor-positive cells. Moreover, transmission of virus between pig-tailed macaque DCs and CD4(+) T cells is largely ptDC-SIGN dependent. Interestingly, MAbs directed against ptDC-SIGN vary in the capacity to block transmission of different SIVmne variants. These data demonstrate that ptDC-SIGN plays a central role in transmitting virus from macaque DCs to T cells, and they suggest that SIVmne variants may differ in their interactions with ptDC-SIGN. Thus, SIVmne infection of pig-tailed macaques may provide an opportunity to investigate the significance of DC-SIGN in primate lentiviral infections.
Figures


Similar articles
-
Domains of macaque DC-SIGN essential for capture and transfer of simian immunodeficiency virus.Virology. 2004 Jun 20;324(1):194-203. doi: 10.1016/j.virol.2004.03.026. Virology. 2004. PMID: 15183066
-
Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells.J Virol. 2002 Sep;76(18):9135-42. doi: 10.1128/jvi.76.18.9135-9142.2002. J Virol. 2002. PMID: 12186897 Free PMC article.
-
Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction.J Immunol. 2005 Oct 1;175(7):4265-73. doi: 10.4049/jimmunol.175.7.4265. J Immunol. 2005. PMID: 16177066 Free PMC article.
-
DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission.Curr Top Microbiol Immunol. 2003;276:31-54. doi: 10.1007/978-3-662-06508-2_2. Curr Top Microbiol Immunol. 2003. PMID: 12797442 Review.
-
DC-SIGN points the way to a novel mechanism for HIV-1 transmission.MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review.
Cited by
-
Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes.Retrovirology. 2007 Jan 30;4:6. doi: 10.1186/1742-4690-4-6. Retrovirology. 2007. PMID: 17263871 Free PMC article.
-
DC-SIGN from African green monkeys is expressed in lymph nodes and mediates infection in trans of simian immunodeficiency virus SIVagm.J Virol. 2004 Jan;78(2):798-810. doi: 10.1128/jvi.78.2.798-810.2004. J Virol. 2004. PMID: 14694112 Free PMC article.
-
Relative replication capacity of phenotypic SIV variants during primary infections differs with route of inoculation.Retrovirology. 2010 Oct 13;7:88. doi: 10.1186/1742-4690-7-88. Retrovirology. 2010. PMID: 20942954 Free PMC article.
-
Innate Immune Response Against HIV-1.Adv Exp Med Biol. 2021;1313:23-58. doi: 10.1007/978-3-030-67452-6_3. Adv Exp Med Biol. 2021. PMID: 34661890
-
ICAM-3 influences human immunodeficiency virus type 1 replication in CD4(+) T cells independent of DC-SIGN-mediated transmission.Virology. 2007 Aug 1;364(2):383-94. doi: 10.1016/j.virol.2007.03.017. Epub 2007 Apr 16. Virology. 2007. PMID: 17434553 Free PMC article.
References
-
- Adema, G. J., F. Hartgers, R. Verstraten, E. de Vries, G. Marland, S. Menon, J. Foster, Y. Xu, P. Nooyen, T. McClanahan, K. B. Bacon, and C. G. Figdor. 1997. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 387:713-717. - PubMed
-
- Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pig-tailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. - PMC - PubMed
-
- Bell, D., J. W. Young, and J. Banchereau. 1999. Dendritic cells. Adv. Immunol. 72:255-324. - PubMed
-
- Cameron, P., M. Pope, A. Granelli-Piperno, and R. M. Steinman. 1996. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 59:158-171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

