Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase
- PMID: 12414936
- PMCID: PMC136897
- DOI: 10.1128/jvi.76.23.11943-11952.2002
Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase
Abstract
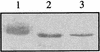
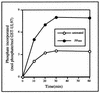
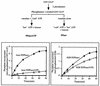
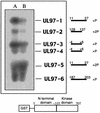
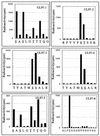
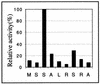
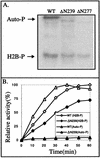
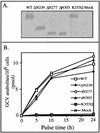
Human cytomegalovirus encodes an unusual protein kinase, UL97, which is a member of the HvU(L) family of protein kinases encoded by diverse herpesviruses. UL97 is able to autophosphorylate and to phosphorylate certain exogenous substrates, including nucleoside analogs such as ganciclovir. It has previously been concluded that phosphorylation of UL97 is essential for its phosphorylation of ganciclovir. We examined the relationship between autophosphorylation of UL97 and its activity on exogenous substrates. Glutathione S-transferase-UL97 fusion protein purified from insect cells was found to be already partially phosphorylated, but neither extensive autophosphorylation nor phosphatase treatment meaningfully altered the time course of its phosphorylation of the exogenous substrate, histone H2B. Sequencing and mass spectrometric analyses of (32)P-labeled tryptic peptides of the UL97 fusion protein identified nine sites of autophosphorylation, all within the first 200 residues of the protein, outside of conserved protein kinase subdomains. A peptide corresponding to the N-terminal UL97 segment that was most extensively autophosphorylated was readily phosphorylated by UL97, confirming that fusion protein sequences are not required for phosphorylation at this site. Deletion mutants lacking at least the first 239 residues exhibited drastically reduced autophosphorylation (<5%) but retained near-wild-type H2B phosphorylation activity. Baculoviruses expressing these mutants efficiently directed the phosphorylation of ganciclovir in insect cells. Taken together, these results identify the autophosphorylation sites of a herpesvirus protein kinase and show that autophosphorylation of UL97 is not required for phosphorylation of exogenous substrates.
Figures

References
-
- Baek, M.-C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase: importance of the P+5 position. J. Biol. Chem. 277:29593-29599. - PubMed
-
- Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. - PMC - PubMed
-
- Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta-, and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. - PubMed
-
- Crossen, R., and S. Gruenwald. 1998. Baculovirus expression vector system manual, 5th ed. Pharmingen, San Diego, Calif.
-
- Dadd, C. A., R. G. Cook, and C. D. Allis. 1993. Fractionation of small tryptic phosphopeptides by alkaline PAGE followed by amino acid sequencing. BioTechniques 14:266-273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

