Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence
- PMID: 12414951
- PMCID: PMC136885
- DOI: 10.1128/jvi.76.23.12097-12111.2002
Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence
Abstract
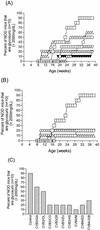
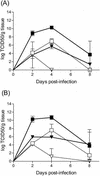
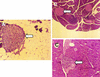
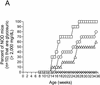
Insulin-dependent (type 1) diabetes mellitus (T1D) onset is mediated by individual human genetics as well as undefined environmental influences such as viral infections. The group B coxsackieviruses (CVB) are commonly named as putative T1D-inducing agents. We studied CVB replication in nonobese diabetic (NOD) mice to assess how infection by diverse CVB strains affected T1D incidence in a model of human T1D. Inoculation of 4- or 8-week-old NOD mice with any of nine different CVB strains significantly reduced the incidence of T1D by 2- to 10-fold over a 10-month period relative to T1D incidences in mock-infected control mice. Greater protection was conferred by more-pathogenic CVB strains relative to less-virulent or avirulent strains. Two CVB3 strains were employed to further explore the relationship of CVB virulence phenotypes to T1D onset and incidence: a pathogenic strain (CVB3/M) and a nonvirulent strain (CVB3/GA). CVB3/M replicated to four- to fivefold-higher titers than CVB3/GA in the pancreas and induced widespread pancreatitis, whereas CVB3/GA induced no pancreatitis. Apoptotic nuclei were detected by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay in CVB3/M-infected pancreata but not in CVB3/GA-infected pancreata. In situ hybridization detected CVB3 RNA in acinar tissue but not in pancreatic islets. Although islets demonstrated inflammatory infiltrates in CVB3-protected mice, insulin remained detectable by immunohistochemistry in these islets but not in those from diabetic mice. Enzyme-linked immunosorbent assay-based examination of murine sera for immunoglobulin G1 (IgG1) and IgG2a immunoreactivity against diabetic autoantigens insulin and HSP60 revealed no statistically significant relationship between CVB3-protected mice or diabetic mice and specific autoimmunity. However, when pooled sera from CVB3/M-protected mice were used to probe a Western blot of pancreatic proteins, numerous proteins were detected, whereas only one band was detected by sera from CVB3/GA-protected mice. No proteins were detected by sera from diabetic or normal mice. Cumulatively, these data do not support the hypothesis that CVB are causative agents of T1D. To the contrary, CVB infections provide significant protection from T1D onset in NOD mice. Possible mechanisms by which this virus-induced protection may occur are discussed.
Figures





References
-
- Akhtar, N., J. Ni, D. Stromberg, G. L. Rosenthal, N. E. Bowles, and J. A. Towbin. 1999. Tracheal aspirate as a substrate for polymerase chain reaction detection of viral genome in childhood pneumonia and myocarditis. Circulation 99:2011-2018. - PubMed
-
- Arnesjo, B., T. Eden, I. Ihse, E. Nordenfelt, and B. Ursing. 1976. Enterovirus infections in acute pancreatitis: a possible etiological connection. Scand. J. Gastroenterol. 11:645-649. - PubMed
-
- Atkinson, M. A., and E. H. Leiter. 1999. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 5:601-604. - PubMed
-
- Atkinson, M. A., and N. Maclaren. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331:1428-1436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

