An antibody to the putative aphid recognition site on cucumber mosaic virus recognizes pentons but not hexons
- PMID: 12414964
- PMCID: PMC136911
- DOI: 10.1128/jvi.76.23.12250-12258.2002
An antibody to the putative aphid recognition site on cucumber mosaic virus recognizes pentons but not hexons
Abstract
Cucumber mosaic virus (CMV), the type member of the genus Cucumovirus (family Bromoviridae), is transmitted by aphids in a nonpersistent manner. Mutagenesis experiments identified the betaH-betaI loop of the capsid subunit as a potential key motif responsible for interactions with the insect vector. To further examine the functional characteristics of this motif, we generated monoclonal antibodies that bound to native virions but not to betaH-betaI mutants. Fab fragments from these antibodies were complexed with wild-type CMV and the virus-Fab structure was determined to 12-A resolution by using electron cryomicroscopy and image reconstruction techniques. The electron density attributed to the bound antibody has a turret-like appearance and protrudes from each of the 12 fivefold axes of the icosahedral virus. Thus, the antibody binds only to the pentameric clusters (pentons) of A subunits of the T=3 quasisymmetric virus and does not appear to bind to any of the B and C subunits that occur as hexameric clusters (hexons) at the threefold (quasi-sixfold) axes. Modeling and electron density comparisons were used to analyze the paratope-epitope interface and demonstrated that the antibody binds to three betaH-betaI loops in three adjacent A subunits in each penton. This antibody can discriminate between A and B/C subunits even though the betaH-betaI loop adopts the same structure in all 180 capsid subunits and is therefore recognizing differences in subunit arrangements. Antibodies with such character have potential use as probes of viral assembly. Our results may provide an additional rationale for designing synthetic vaccines by using symmetrical viral particles.
Figures



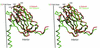



References
-
- Bailey, S. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50:760-763. - PubMed
-
- Baker, T. S., and R. H. Cheng. 1996. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J. Struct. Biol. 116:120-130. - PubMed
-
- Booy, F. P., R. B. S. Roden, H. L. Greenstone, J. T. Schiller, and B. L. Trus. 1998. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 Å resolution. J. Mol. Biol. 281:95-106. - PubMed
-
- Burke, K. L., G. Dunn, M. Ferguson, P. Minor, and J. W. Almond. 1988. Antigen chimeras of poliovirus as potential vaccines. Nature 332:81-82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

