Antibody epitopes on the neuraminidase of a recent H3N2 influenza virus (A/Memphis/31/98)
- PMID: 12414967
- PMCID: PMC136895
- DOI: 10.1128/jvi.76.23.12274-12280.2002
Antibody epitopes on the neuraminidase of a recent H3N2 influenza virus (A/Memphis/31/98)
Abstract
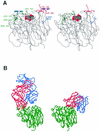
We have characterized monoclonal antibodies raised against the neuraminidase (NA) of a Sydney-like influenza virus (A/Memphis/31/98, H3N2) in a reassortant virus A/NWS/33(HA)-A/Mem/31/98(NA) (H1N2) and nine escape mutants selected by these monoclonal antibodies. Five of the antibodies use the same heavy chain VDJ genes and may not be independent. Another antibody, Mem5, uses the same V(H) and J genes with a different D gene and different isotype. Sequence changes in escape mutants selected by these antibodies occur in two loops of the NA, at amino acid 198, 199, 220, or 221. These amino acids are located on the opposite side of the NA monomer to the major epitopes found in N9 and early N2 NAs. Escape mutants with a change at 198 have reduced NA activity compared to the wild-type virus. Asp198 points toward the substrate binding pocket, and we had previously found that a site-directed mutation of this amino acid resulted in a loss of enzyme activity (M. R. Lentz, R. G. Webster, and G. M. Air, Biochemistry 26:5351-5358, 1987). Mutations at residue 199, 220, or 221 did not alter the NA activity significantly compared to that of wild-type NA. A 3.5-A structure of Mem5 Fab complexed with the Mem/98 NA shows that the Mem5 antibody binds at the sites of escape mutation selected by the other antibodies.
Figures



References
-
- Air, G. M., M. C. Els, L. E. Brown, W. G. Laver, and R. G. Webster. 1985. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology 145:237-248. - PubMed
-
- Air, G. M., W. G. Laver, M. Luo, S. J. Stray, G. Legrone, and R. G. Webster. 1990. Antigenic, sequence and crystal variation in influenza B neuraminidase. Virology 177:578-587. - PubMed
-
- Blok, J., and G. M. Air. 1982. Variation in the membrane-insertion and “stalk” sequences in eight subtypes of influenza type A virus neuraminidase. Biochemistry 21:4001-4007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

