Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice
- PMID: 12414974
- PMCID: PMC136916
- DOI: 10.1128/jvi.76.23.12335-12343.2002
Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice
Abstract
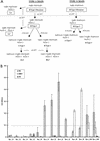
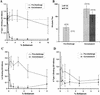
Attachment of Norwalk (NV), Snow Mountain (SMV), and Hawaii (HV) virus-like particles (VLPs) to specific ABH histo-blood group antigens was investigated by using human saliva and synthetic biotinylated carbohydrates. The three distinct Norwalk-like viruses (NLVs) have various capacities for binding ABH histo-blood group antigens, suggesting that different mechanisms for NLV attachment likely exist. Importantly, antisera from NV-infected human volunteers, as well as from mice inoculated with packaged Venezuelan equine encephalitis virus replicons expressing NV VLPs, blocked the ability of NV VLPs to bind synthetic H type 1, Le(b), and H type 3, suggesting a potential mechanism for antibody-mediated neutralization of NV.
Figures




References
-
- Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. K. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 117:40-48. - PubMed
-
- Edwards, M. J., and N. J. Dimmock. 2001. A haemagglutinin (HA1)-specific FAb neutralizes influenza A virus by inhibiting fusion activity. J. Gen. Virol. 82:1387-1395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

