Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease?
- PMID: 12415119
- PMCID: PMC137541
- DOI: 10.1073/pnas.232588799
Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease?
Abstract
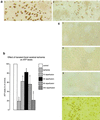
Ubiquitylated protein aggregates are characteristic features of neurodegenerative disorders that are also found in acute pathological states of the brain such as stroke. Many of the proteins connected to neurodegenerative diseases play a role in the ubiquitin-proteasomal pathway. Mutation of one of these proteins, the E3 ubiquitin ligase parkin, is the cause of autosomal recessive juvenile Parkinson's disease. Here we show that transient focal cerebral ischemia of 1-h duration induces marked depletion of parkin protein levels, to 60%, 36%, 33%, and 25% of controls after 1, 3, 6, and 24 h of reperfusion, but that ischemia does not cause lower protein levels of E2 ubiquitin-conjugating enzymes Ubc6, Ubc7, or Ubc9. After 3 h of reperfusion, when parkin protein levels were already reduced to <40% of control, ATP levels were almost completely recovered from ischemia and we did not observe DNA fragmentation, suggesting that parkin depletion preceded development of neuronal cell death. Up-regulation of the expression of parkin has been shown to protect cells from injury induced by endoplasmic reticulum (ER) dysfunction, and this form of cellular stress is also triggered by transient cerebral ischemia. However, in contrast to observations in neuroblastoma cells, we saw no up-regulation of parkin expression in primary neuronal cell cultures after induction of ER dysfunction. Our data thus suggest that ischemia-induced depletion of parkin protein may contribute to the pathological process resulting in cell injury by increasing the sensitivity of neurons to ER dysfunction and the aggregation of ubiquitylated proteins during the reperfusion period.
Figures

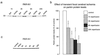

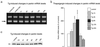
References
-
- Kleihues P., Hossmann, K.-A., Pegg, A. E., Kobayashi, K. & Zimmermann, V. (1975) Brain Res. 95, 61-73. - PubMed
-
- Cooper H. K., Zalewska, T., Kawakami, S. & Hossmann, K.-A. (1977) J. Neurochem. 28, 929-934. - PubMed
-
- Kumar R., Azam, S., Sullivan, J. M., Owen, C., Cavener, D. R. C., Zhang, P., Ron, D., Harding, H. P., Chen, J.-J., Han, A., et al. (2001) J. Neurochem. 77, 1418-1421. - PubMed
-
- Harding H. P., Zhang, Y. & Ron, D. (1999) Nature 397, 271-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

