Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions
- PMID: 12417558
- PMCID: PMC151620
- DOI: 10.1172/JCI16781
Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions
Figures

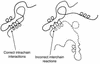
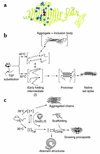
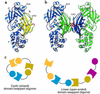


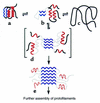

References
-
- Weatherall, D.J., Clegg, J.B., Higgs, D.R., and Wood, W.G. 2001. The hemoglobinopathies. In Metabolic and molecular bases of inherited disease. C. Scriver, A. Beaudet, D. Valle, and W. Sly, editors. McGraw-Hill. New York, New York, USA. 4571–4636.
-
- Cox, D.W. 2001. α1-Antitrypsin deficiency. In Metabolic and molecular bases of inherited disease. C. Scriver, A. Beaudet, D. Valle, and W. Sly, editors. McGraw-Hill. New York, New York, USA. 5559–5584.
-
- Selkoe, D. 1997. Cellular and molecular biology of the beta-amyloid precursor protein and Alzheimer’s disease. In The molecular and genetic basis of neurological disease. R.N. Rosenberg, S.B. Prusiner, S. DiMauro, and R.L. Barchi, editors. Butterworth-Heinemann. Boston, Massachusetts, USA. 601–611.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

