Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications
- PMID: 12417562
- PMCID: PMC151610
- DOI: 10.1172/JCI15736
Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications
Abstract
The wealth of clinical epidemiological data on the association between intra-abdominal fat accumulation and morbidity sharply contrasts with the paucity of knowledge about the determinants of fat distribution, which cannot be explained merely in terms of humoral factors. If it comes to neuronal control, until now, adipose tissue was reported to be innervated by the sympathetic nervous system only, known for its catabolic effect. We hypothesized the presence of a parasympathetic input stimulating anabolic processes in adipose tissue. Intra-abdominal fat pads in rats were first sympathetically denervated and then injected with the retrograde transneuronal tracer pseudorabies virus (PRV). The resulting labeling of PRV in the vagal motor nuclei of the brain stem reveals that adipose tissue receives vagal input. Next, we assessed the physiological impact of these findings by combining a fat pad-specific vagotomy with a hyperinsulinemic euglycemic clamp and RT-PCR analysis. Insulin-mediated glucose and FFA uptake were reduced by 33% and 36%, respectively, whereas the activity of the catabolic enzyme hormone-sensitive lipase increased by 51%. Moreover, expression of resistin and leptin mRNA decreased, whereas adiponectin mRNA did not change. All these data indicate an anabolic role for the vagal input to adipose tissue. Finally, we demonstrate somatotopy within the central part of the autonomic nervous system, as intra-abdominal and subcutaneous fat pads appeared to be innervated by separate sympathetic and parasympathetic motor neurons. In conclusion, parasympathetic input to adipose tissue clearly modulates its insulin sensitivity and glucose and FFA metabolism in an anabolic way. The implications of these findings for the (patho)physiology of fat distribution are discussed.
Figures

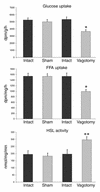
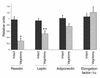
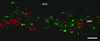

Comment in
-
Dual innervation of white adipose tissue: some evidence for parasympathetic nervous system involvement.J Clin Invest. 2002 Nov;110(9):1235-7. doi: 10.1172/JCI17047. J Clin Invest. 2002. PMID: 12417560 Free PMC article. No abstract available.
References
-
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. - PubMed
-
- Kalsbeek A, et al. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. - PubMed
-
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. - PubMed
-
- Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59:719–809. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

