Hereditary sensory neuropathy type 1 mutations confer dominant negative effects on serine palmitoyltransferase, critical for sphingolipid synthesis
- PMID: 12417569
- PMCID: PMC151618
- DOI: 10.1172/JCI16450
Hereditary sensory neuropathy type 1 mutations confer dominant negative effects on serine palmitoyltransferase, critical for sphingolipid synthesis
Abstract
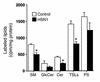
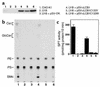
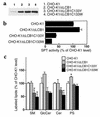
Hereditary sensory neuropathy type 1 (HSN1) is a dominantly inherited degenerative disorder of the peripheral nerves. HSN1 is clinically and genetically heterogeneous. One form arises from mutations in the gene SPTLC1 encoding long-chain base 1 (LCB1), one of two subunits of serine palmitoyltransferase (SPT), the enzyme catalyzing the initial step of sphingolipid synthesis. We have examined the effects of the mutations C133Y and C133W, which we have identified in two HSN1 families, on the function of SPT. Although in HSN1 lymphoblasts, the C133Y and C133W mutations do not alter the steady-state levels of LCB1 and LCB2 subunits, they result in reduced SPT activity and sphingolipid synthesis. Moreover, in a mutant Chinese hamster ovary (CHO) cell strain with defective SPT activity due to a lack of the LCB1 subunit, these mutations impair the ability of the LCB1 subunit to complement the SPT deficiency. Furthermore, the overproduction of either the LCB1C133Y or LCB1C133W subunit inhibits SPT activity in CHO cells despite the presence of wild-type LCB1. In addition, we demonstrate that in CHO cells the mutant LCB1 proteins, similar to the normal LCB1, can interact with the wild-type LCB2 subunit. These results indicate that the HSN1-associated mutations in LCB1 confer dominant negative effects on the SPT enzyme.
Figures
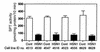


References
-
- Whitaker JN, Falchuck ZM, Engel WK, Blaese RM, Strober W. Hereditary sensory neuropathy. Association with increased synthesis of immunoglobulin A. Arch Neurol. 1974;30:359–371. - PubMed
-
- Dyck, P.J. 1993. Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neuron. In Peripheral neuropathies. P.J. Dyck, P.K. Thomas, J.W. Griffin, P.A. Low, and J.F. Poduslo, editors. W.B. Saunders Co., Philadelphia, Pennsylvania, USA. 1065–1093.
-
- Hicks EP. Hereditary perforating ulcers of the feet. Lancet. 1922;1:319–321.
-
- Hageman G, Hilhorst BG, Rozeboom AR. Is there involvement of the central nervous system in hereditary sensory radicular neuropathy? Clin Neurol Neurosurg. 1992;94:49–54. - PubMed

