Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo
- PMID: 12417570
- PMCID: PMC151606
- DOI: 10.1172/JCI15451
Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo
Abstract
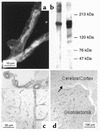
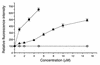
Paclitaxel concentrations in the brain are very low after intravenous injection. Since paclitaxel is excluded from some tumors by p-glycoprotein (p-gp), the same mechanism may prevent entry into the brain. In vitro, paclitaxel transport was examined in capillaries from rat brains by confocal microscopy using BODIPY Fl-paclitaxel. Western blots and immunostaining demonstrated apical expression of p-gp in isolated endothelial cells, vessels, and tissue. Secretion of BODIPY Fl-paclitaxel into capillary lumens was specific and energy-dependent. Steady state luminal fluorescence significantly exceeded cellular fluorescence and was reduced by NaCN, paclitaxel, and SDZ PSC-833 (valspodar), a p-gp blocker. Leukotriene C(4) (LTC(4)), an Mrp2-substrate, had no effect. Luminal accumulation of NBDL-cyclosporin, a p-gp substrate, was inhibited by paclitaxel. In vivo, paclitaxel levels in the brain, liver, kidney, and plasma of nude mice were determined after intravenous injection. Co-administration of valspodar led to increased paclitaxel levels in brains compared to monotherapy. Therapeutic relevance was proven for nude mice with implanted intracerebral human U-118 MG glioblastoma. Whereas paclitaxel did not affect tumor volume, co-administration of paclitaxel (intravenous) and PSC833 (peroral) reduced tumor volume by 90%. Thus, p-gp is an important obstacle preventing paclitaxel entry into the brain, and inhibition of this transporter allows the drug to reach sensitive tumors within the CNS.
Figures
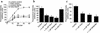



References
-
- Kroger N, Achterrath W, Hegewisch-Becker S, Mross K, Zander AR. Current options in treatment of anthracycline-resistant breast cancer. Cancer Treat Rev. 1999;25:279–291. - PubMed
-
- Nathan FE, Berd D, Sato T, Mastrangelo MJ. Paclitaxel and tamoxifen: an active regimen for patients with metastatic melanoma. Cancer. 2000;88:79–87. - PubMed
-
- Muggia FM, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106–115. - PubMed
-
- Papadimitriou CA. Paclitaxel, cisplatin, and epirubicin first-line chemotherapy in stage III and IV ovarian carcinoma: long-term results of a phase II study. Cancer. 2000;89:1547–1554. - PubMed
-
- Belani CP. Paclitaxel and docetaxel combinations in non-small cell lung cancer. Chest. 2000;117(Suppl 1):144S–151S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

