Visualization of the intracellular behavior of HIV in living cells
- PMID: 12417576
- PMCID: PMC2173076
- DOI: 10.1083/jcb.200203150
Visualization of the intracellular behavior of HIV in living cells
Abstract
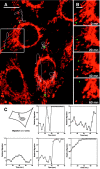
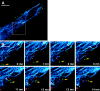
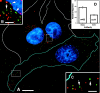
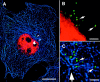
To track the behavior of human immunodeficiency virus (HIV)-1 in the cytoplasm of infected cells, we have tagged virions by incorporation of HIV Vpr fused to the GFP. Observation of the GFP-labeled particles in living cells revealed that they moved in curvilinear paths in the cytoplasm and accumulated in the perinuclear region, often near the microtubule-organizing center. Further studies show that HIV uses cytoplasmic dynein and the microtubule network to migrate toward the nucleus. By combining GFP fused to the NH2 terminus of HIV-1 Vpr tagging with other labeling techniques, it was possible to determine the state of progression of individual particles through the viral life cycle. Correlation of immunofluorescent and electron micrographs allowed high resolution imaging of microtubule-associated structures that are proposed to be reverse transcription complexes. Based on these observations, we propose that HIV uses dynein and the microtubule network to facilitate the delivery of the viral genome to the nucleus of the cell during early postentry steps of the HIV life cycle.
Figures



Comment in
-
Unchain my heart, baby let me go--the entry and intracellular transport of HIV.J Cell Biol. 2002 Nov 11;159(3):393-5. doi: 10.1083/jcb.200210024. Epub 2002 Nov 11. J Cell Biol. 2002. PMID: 12427864 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

