Reduced self-reactivity of an autoreactive T cell after activation with cross-reactive non-self-ligand
- PMID: 12417626
- PMCID: PMC2194103
- DOI: 10.1084/jem.20020390
Reduced self-reactivity of an autoreactive T cell after activation with cross-reactive non-self-ligand
Abstract
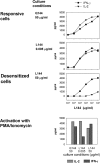
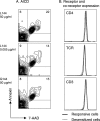
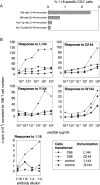
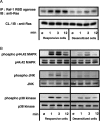
Autoreactive CD4(+) T lymphocytes are critical to the induction of autoimmune disease, but because of the degenerate nature of T cell receptor (TCR) activation such receptors also respond to other ligands. Interaction of autoreactive T cells with other non-self-ligands has been shown to activate and expand self-reactive cells and induce autoimmunity. To understand the effect on the autoreactivity of naive cross-reactive T cells of activation with a potent nonself ligand, we have generated a TCR transgenic mouse which expresses a TCR with a broad cross-reactivity to a number of ligands including self-antigen. The activation of naive transgenic recombination activating gene (Rag)2(-)(/)(-) T cells with a potent non-self-ligand did not result in a enhancement of reactivity to self, but made these T cells nonresponsive to the self-ligand and anti-CD3, although they retained a degree of responsiveness to the non-self-ligand. These desensitized cells had many characteristics of anergic T cells. Interleukin (IL)-2 production was selectively reduced compared with interferon (IFN)-gamma. p21(ras) activity was reduced and p38 mitogen-activated protein kinase (MAPK) was relatively spared, consistent with known biochemical characteristics of anergy. Surprisingly, calcium fluxes were also affected and the anergic phenotype could not be reversed by exogenous IL-2. Therefore, activation with a hyperstimulating non-self-ligand changes functional specificity of an autoreactive T cell without altering the TCR. This mechanism may preserve the useful reactivity of peripheral T cells to foreign antigen while eliminating responses to self.
Figures




Similar articles
-
A population of in vivo anergized T cells with a lower activation threshold for the induction of CD25 exhibit differential requirements in mobilization of intracellular calcium and mitogen-activated protein kinase activation.J Immunol. 2000 Mar 15;164(6):2881-9. doi: 10.4049/jimmunol.164.6.2881. J Immunol. 2000. PMID: 10706673
-
Constitutive active p21ras enhances primary T cell responsiveness to Ca2+ signals without interfering with the induction of clonal anergy.Eur J Immunol. 2002 Sep;32(9):2500-9. doi: 10.1002/1521-4141(200209)32:9<2500::AID-IMMU2500>3.0.CO;2-S. Eur J Immunol. 2002. PMID: 12207334
-
Contributions of the T cell receptor-associated CD3gamma-ITAM to thymocyte selection.J Exp Med. 2002 Jul 1;196(1):1-13. doi: 10.1084/jem.20020268. J Exp Med. 2002. PMID: 12093866 Free PMC article.
-
Modification of human T-cell responses by altered peptide ligands: a new approach to antigen-specific modification.Intern Med. 1998 Oct;37(10):804-17. doi: 10.2169/internalmedicine.37.804. Intern Med. 1998. PMID: 9840700 Review.
-
The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation.Annu Rev Immunol. 1999;17:467-522. doi: 10.1146/annurev.immunol.17.1.467. Annu Rev Immunol. 1999. PMID: 10358766 Review.
Cited by
-
Epi-allelic Erk1 and Erk2 knockdown series for quantitative analysis of T cell Erk regulation and IL-2 production.Mol Immunol. 2007 May;44(12):3085-91. doi: 10.1016/j.molimm.2007.02.008. Epub 2007 Apr 5. Mol Immunol. 2007. PMID: 17418417 Free PMC article.
-
Manipulating antigenic ligand strength to selectively target myelin-reactive CD4+ T cells in EAE.J Neuroimmune Pharmacol. 2010 Jun;5(2):176-88. doi: 10.1007/s11481-009-9181-3. Epub 2009 Nov 11. J Neuroimmune Pharmacol. 2010. PMID: 19904613 Free PMC article. Review.
-
Transforming Boolean models to continuous models: methodology and application to T-cell receptor signaling.BMC Syst Biol. 2009 Sep 28;3:98. doi: 10.1186/1752-0509-3-98. BMC Syst Biol. 2009. PMID: 19785753 Free PMC article.
-
Biofilm of Klebsiella pneumoniae minimize phagocytosis and cytokine expression by macrophage cell line.AMB Express. 2022 Sep 19;12(1):122. doi: 10.1186/s13568-022-01465-z. AMB Express. 2022. PMID: 36121578 Free PMC article.
-
The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance.PLoS Biol. 2006 Oct;4(11):e340. doi: 10.1371/journal.pbio.0040340. PLoS Biol. 2006. PMID: 17048986 Free PMC article.
References
-
- Kersh, G.J., and P.M. Allen. 1996. Essential flexibility in the T-cell antigen recognition of antigen. Nature. 380:495–498. - PubMed
-
- Mason, D. 1998. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today. 19:395–404. - PubMed
-
- Bevan, M.J. 1997. In thymic selection, peptide diversity gives and takes away. Immunity. 7:175–178. - PubMed
-
- Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. - PubMed
-
- Zamvil, S.S., and L. Steinman. 1990. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8:579–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

