Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation
- PMID: 12417650
- PMCID: PMC6758018
- DOI: 10.1523/JNEUROSCI.22-21-09244.2002
Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation
Abstract
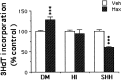
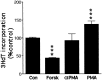
Although positive and negative signals control neurogenesis in the embryo, factors regulating postnatal proliferation are less well characterized. In the vertebrate cerebellum, Sonic Hedgehog (Shh) is an efficacious mitogen for cerebellar granule neuron precursors (GNPs), and mutations activating the Shh pathway are linked to medulloblastoma, a tumor derived from GNPs. Although the mitogenic effects of Shh can be blocked by increasing cAMP or protein kinase A activity, the physiological factors antagonizing this stimulation are undefined. In the embryo, pituitary adenylate cyclase-activating polypeptide (PACAP) receptor 1 (PAC1) signaling regulates neural precursor proliferation. We now show that in the developing cerebellum, PAC1 mRNA colocalizes with gene transcripts for Shh receptor Patched 1 and target gene Gli1 in the external germinal layer. We consequently investigated the interactions of PACAP and Shh in proliferation of purified GNPs in culture. Shh exhibited mitogenic activity in both rat and mouse cultures, stimulating DNA synthesis approximately 10-fold after 48 hr of exposure. PACAP markedly inhibited Shh-induced thymidine incorporation by 50 and 85% in rat and mouse GNPs, respectively, but did not significantly affect the stimulation induced by other mitogens. This selective effect was reproduced by the specific PAC1 agonist maxadilan, as well as by the adenylate cyclase activator forskolin, suggesting that PAC1 provides a potent inhibitory signal for Shh-induced proliferation in developing cerebellum. In contrast, in the absence of Shh, PACAP and maxadilan modestly stimulated DNA synthesis, an effect reproduced by activating protein kinase C. These observations suggest that G-protein-coupled receptors, such as PAC1, serve as sensors of environmental cues, coordinating diverse neurogenetic signals.
Figures
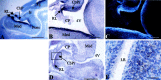
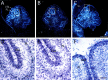


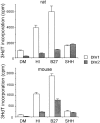
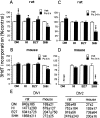
Similar articles
-
Pituitary adenylyl cyclase activating polypeptide inhibits gli1 gene expression and proliferation in primary medulloblastoma derived tumorsphere cultures.BMC Cancer. 2010 Dec 9;10:676. doi: 10.1186/1471-2407-10-676. BMC Cancer. 2010. PMID: 21143927 Free PMC article.
-
PACAP promotes neural stem cell proliferation in adult mouse brain.J Neurosci Res. 2004 Apr 15;76(2):205-15. doi: 10.1002/jnr.20038. J Neurosci Res. 2004. PMID: 15048918
-
Up-regulation of the PACAP type-1 receptor (PAC1) promoter by neurotrophins in rat PC12 cells and mouse cerebellar granule cells via the Ras/mitogen-activated protein kinase cascade.J Neurochem. 2002 Sep;82(5):1199-207. doi: 10.1046/j.1471-4159.2002.01124.x. J Neurochem. 2002. PMID: 12358767
-
[Physiological significance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the nervous system].Yakugaku Zasshi. 2002 Dec;122(12):1109-21. doi: 10.1248/yakushi.122.1109. Yakugaku Zasshi. 2002. PMID: 12510388 Review. Japanese.
-
[Pituitary adenylate cyclase-activating polypeptide].Ann Endocrinol (Paris). 1998 Dec;59(5):364-405. Ann Endocrinol (Paris). 1998. PMID: 9949891 Review. French.
Cited by
-
Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA.Cell Signal. 2013 Nov;25(11):2222-30. doi: 10.1016/j.cellsig.2013.07.012. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872071 Free PMC article.
-
Differential modulation of Sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network.Dev Neurosci. 2010 Mar;32(1):59-70. doi: 10.1159/000274458. Epub 2010 Mar 25. Dev Neurosci. 2010. PMID: 20389077 Free PMC article.
-
PACAP and migraine headache: immunomodulation of neural circuits in autonomic ganglia and brain parenchyma.J Headache Pain. 2018 Mar 13;19(1):23. doi: 10.1186/s10194-018-0850-6. J Headache Pain. 2018. PMID: 29536279 Free PMC article. Review.
-
PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors.J Neurosci. 2004 Nov 17;24(46):10343-52. doi: 10.1523/JNEUROSCI.3229-04.2004. J Neurosci. 2004. PMID: 15548648 Free PMC article.
-
Expression of the human PAC1 receptor leads to dose-dependent hydrocephalus-related abnormalities in mice.J Clin Invest. 2006 Jul;116(7):1924-34. doi: 10.1172/JCI27597. J Clin Invest. 2006. PMID: 16823490 Free PMC article.
References
-
- Akimaru H, Chen Y, Dai P, Hou DX, Nonaka M, Smolik SM, Armstrong S, Goodman RH, Ishii S. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature. 1997;386:735–738. - PubMed
-
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure and functions. CRC; New York: 1996.
-
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
