Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein
- PMID: 12417659
- PMCID: PMC6758022
- DOI: 10.1523/JNEUROSCI.22-21-09340.2002
Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein
Abstract
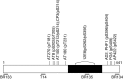
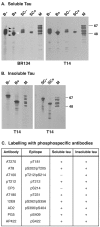
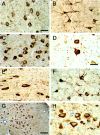
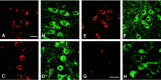
The identification of mutations in the Tau gene in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) has made it possible to express human tau protein with pathogenic mutations in transgenic animals. Here we report on the production and characterization of a line of mice transgenic for the 383 aa isoform of human tau with the P301S mutation. At 5-6 months of age, homozygous animals from this line developed a neurological phenotype dominated by a severe paraparesis. According to light microscopy, many nerve cells in brain and spinal cord were strongly immunoreactive for hyperphosphorylated tau. According to electron microscopy, abundant filaments made of hyperphosphorylated tau protein were present. The majority of filaments resembled the half-twisted ribbons described previously in cases of FTDP-17, with a minority of filaments resembling the paired helical filaments of Alzheimer's disease. Sarkosyl-insoluble tau from brains and spinal cords of transgenic mice ran as a hyperphosphorylated 64 kDa band, the same apparent molecular mass as that of the 383 aa tau isoform in the human tauopathies. Perchloric acid-soluble tau was also phosphorylated at many sites, with the notable exception of serine 214. In the spinal cord, neurodegeneration was present, as indicated by a 49% reduction in the number of motor neurons. No evidence for apoptosis was obtained, despite the extensive colocalization of hyperphosphorylated tau protein with activated MAP kinase family members. The latter may be involved in the hyperphosphorylation of tau.
Figures







References
-
- Andreadis A, Brown MW, Kosik K. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. - PubMed
-
- Atzori C, Ghetti B, Piva R, Srinivasan AN, Zolo P, Delisle MB, Mirra SS, Migheli A. Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J Neuropathol Exp Neurol. 2001;60:1190–1197. - PubMed
-
- Buée-Scherrer V, Condamines O, Mourton-Gilles C, Jakes R, Goedert M, Pau B, Delacourte A. AD2, a phosphorylation-dependent monoclonal antibody directed against tau proteins found in Alzheimer's disease. Brain Res Mol Brain Res. 1996;39:79–88. - PubMed
-
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999;58:667–677. - PubMed
-
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz-50's selectivity for Alzheimer's disease pathology. J Biol Chem. 1996;271:32789–32795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
