Growth cone turning induced by direct local modification of microtubule dynamics
- PMID: 12417661
- PMCID: PMC6758015
- DOI: 10.1523/JNEUROSCI.22-21-09358.2002
Growth cone turning induced by direct local modification of microtubule dynamics
Abstract
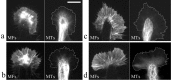
Pathfinding by nerve growth cones depends on attractive and repulsive turning in response to a variety of guidance cues. Here we present direct evidence to demonstrate an essential and instructive role for microtubules (MTs) in growth cone steering. First, both growth cone attraction and repulsion induced by diffusible cues in culture can be completely blocked by low concentrations of drugs that specifically inhibit dynamic microtubule ends in the growth cone. Second, direct focal photoactivated release of the microtubule-stabilizing drug taxol on one side of the growth cone consistently induces attraction (turning toward the site of application). Using the focal pipette application method, we also show that local MT stabilization by taxol induces growth cone attraction, whereas local MT destabilization by the microtubule-disrupting drug nocodazole induces repulsion (turning away). Finally, the microtubule-initiated attractive turning requires the participation of the actin cytoskeleton: local microtubule stabilization induces preferential protrusion of lamellipodia before the attractive turning, and the attraction can be abolished by inhibition of either actin polymerization or the Rho family GTPases. Together, these results demonstrate a novel steering mechanism for growth cones in which local and selective modification of dynamic microtubules can initiate and instruct directional steering. With the subsequent concerted activity of the actin cytoskeleton, this microtubule-initiated mechanism provides the growth cone with the additional means to efficiently navigate through its environment.
Figures




References
-
- Bentley D, O'Connor T. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–48. - PubMed
-
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. - PubMed
-
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
