Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ
- PMID: 12417668
- PMCID: PMC6758057
- DOI: 10.1523/JNEUROSCI.22-21-09430.2002
Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ
Abstract
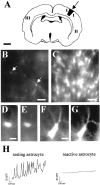
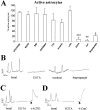
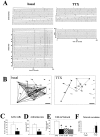
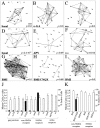
Spontaneous neuronal activity is essential to neural development. Until recently, neurons were believed to be the only excitable cells to display spontaneous activity. However, cultured astrocytes and, more recently, astrocytes in situ are now known to exhibit spontaneous Ca2+ transients. Here we used Ca2+ imaging of astrocytes from transgenic mice for the simultaneous monitoring of [Ca2+]i changes in large numbers of astrocytes. We found that spontaneous activity is a common property of most brain astrocytes that is lost in response to a lesion. These spontaneous [Ca2+]i oscillations require extracellular and intracellular Ca2+. Moreover, network analysis revealed that most astrocytes formed correlated networks of dozens of these cells, which were synchronous with both astrocytes and neurons. We found that decreasing spontaneous [Ca2+]i transients in neurons by TTX does not alter the number of active astrocytes, although it impairs their synchronous network activity. Conversely, bicuculline-induced epileptic patterns of [Ca2+]i transients in neurons cause an increase in the number of active astrocytes and in their network synchrony. Furthermore, activation of non-NMDA and NMDA ionotropic glutamate receptors is required to correlate astrocytic networks. These results show that spontaneous activity in astrocytes and neurons is patterned into correlated neuronal/astrocytic networks in which neuronal activity regulates the network properties of astrocytes. This network activity may be essential for neural development and synaptic plasticity.
Figures



References
-
- Albowitz B, König P, Kuhnt U. Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J Neurophysiol. 1997;77:491–501. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
