Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task
- PMID: 12417672
- PMCID: PMC6758024
- DOI: 10.1523/JNEUROSCI.22-21-09475.2002
Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task
Abstract
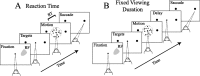
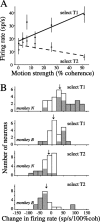
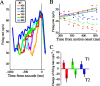
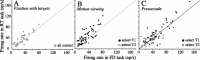
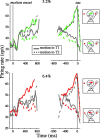
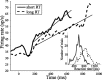
Decisions about the visual world can take time to form, especially when information is unreliable. We studied the neural correlate of gradual decision formation by recording activity from the lateral intraparietal cortex (area LIP) of rhesus monkeys during a combined motion-discrimination reaction-time task. Monkeys reported the direction of random-dot motion by making an eye movement to one of two peripheral choice targets, one of which was within the response field of the neuron. We varied the difficulty of the task and measured both the accuracy of direction discrimination and the time required to reach a decision. Both the accuracy and speed of decisions increased as a function of motion strength. During the period of decision formation, the epoch between onset of visual motion and the initiation of the eye movement response, LIP neurons underwent ramp-like changes in their discharge rate that predicted the monkey's decision. A steeper rise in spike rate was associated with stronger stimulus motion and shorter reaction times. The observations suggest that neurons in LIP integrate time-varying signals that originate in the extrastriate visual cortex, accumulating evidence for or against a specific behavioral response. A threshold level of LIP activity appears to mark the completion of the decision process and to govern the tradeoff between accuracy and speed of perception.
Figures





Similar articles
-
Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey.J Neurophysiol. 2001 Oct;86(4):1916-36. doi: 10.1152/jn.2001.86.4.1916. J Neurophysiol. 2001. PMID: 11600651
-
Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making.J Neurosci. 2005 Nov 9;25(45):10420-36. doi: 10.1523/JNEUROSCI.4684-04.2005. J Neurosci. 2005. PMID: 16280581 Free PMC article.
-
Representation of accumulating evidence for a decision in two parietal areas.J Neurosci. 2015 Mar 11;35(10):4306-18. doi: 10.1523/JNEUROSCI.2451-14.2015. J Neurosci. 2015. PMID: 25762677 Free PMC article.
-
[Parietal Association Area and Motion Information Processing].Brain Nerve. 2016 Nov;68(11):1335-1343. doi: 10.11477/mf.1416200597. Brain Nerve. 2016. PMID: 27852024 Review. Japanese.
-
Multimodal activity in the parietal cortex.Hear Res. 2009 Dec;258(1-2):100-5. doi: 10.1016/j.heares.2009.01.011. Epub 2009 Feb 6. Hear Res. 2009. PMID: 19450431 Free PMC article. Review.
Cited by
-
Two views on the cognitive brain.Nat Rev Neurosci. 2021 Jun;22(6):359-371. doi: 10.1038/s41583-021-00448-6. Epub 2021 Apr 15. Nat Rev Neurosci. 2021. PMID: 33859408 Review.
-
Neural integrators for decision making: a favorable tradeoff between robustness and sensitivity.J Neurophysiol. 2013 May;109(10):2542-59. doi: 10.1152/jn.00976.2012. Epub 2013 Feb 27. J Neurophysiol. 2013. PMID: 23446688 Free PMC article.
-
From Multisensory Integration to Multisensory Decision-Making.Adv Exp Med Biol. 2024;1437:23-35. doi: 10.1007/978-981-99-7611-9_2. Adv Exp Med Biol. 2024. PMID: 38270851 Review.
-
Non-equilibrium landscape and flux reveal the stability-flexibility-energy tradeoff in working memory.PLoS Comput Biol. 2020 Oct 2;16(10):e1008209. doi: 10.1371/journal.pcbi.1008209. eCollection 2020 Oct. PLoS Comput Biol. 2020. PMID: 33006962 Free PMC article.
-
A Two-Stage Process Model of Sensory Discrimination: An Alternative to Drift-Diffusion.J Neurosci. 2016 Nov 2;36(44):11259-11274. doi: 10.1523/JNEUROSCI.1367-16.2016. J Neurosci. 2016. PMID: 27807167 Free PMC article.
References
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol. 1991a;66:1095–1108. - PubMed
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991b;66:1109–1124. - PubMed
-
- Bracewell RM, Mazzoni P, Barash S, Andersen RA. Motor intention activity in the macaque's lateral intraparietal area. II. Changes of motor plan. J Neurophysiol. 1996;76:1457–1464. - PubMed
-
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci. 1993;10:1157–1169. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
