Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus
- PMID: 12417688
- PMCID: PMC6758050
- DOI: 10.1523/JNEUROSCI.22-21-09626.2002
Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus
Abstract
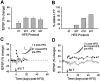
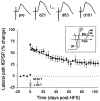
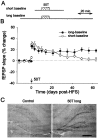
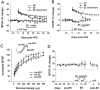
Long-term potentiation (LTP) is widely regarded as a memory mechanism, but it is not known whether it can last long enough to underlie very long-term memory. We report that high-frequency stimulation (HFS) paradigms applied to the rat dentate gyrus can elicit stable LTP lasting months and up to at least 1 year. The induction of stable LTP was sensitive to stimulation variables on the day of HFS and was associated with phosphorylation of cAMP response element-binding protein. The maintenance of stable LTP was also experience-dependent, because it was reversed when animals were exposed repeatedly to an enriched environment beginning 14 d post-HFS. However, stable LTP eventually consolidated over time and became resistant to reversal, because exposure to enriched environments 90 d post-HFS failed to influence stable LTP maintenance. Thus, LTP can be shown to meet one of the principal criteria for a very long-term memory storage mechanism. However, under naturalistic environmental conditions, LTP may normally be retained in the hippocampus for only short periods of time.
Figures

References
-
- Abraham WC, Otani S. Macromolecules and the maintenance of long-term potentiation. In: Morrell F, editor. Kindling and synaptic plasticity. Birkhäuser; Boston: 1991. pp. 92–109.
-
- Abraham WC, Demmer J, Richardson C, Williams J, Lawlor P, Mason SE, Tate WP, Dragunow M. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56:717–727. - PubMed
-
- Abraham WC, Mason-Parker SE, Williams J, Dragunow M. Analysis of the decremental nature of LTP in the dentate gyrus. Mol Brain Res. 1995;30:367–372. - PubMed
-
- Abraham WC, Logan BL, Greenwood J, Dragunow M. CREB phosphorylation-associated stable LTP lasting months in the hippocampus. Proc Int Australas Winter Conf Brain Res. 2001;19:41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
