Transcriptional activating regions target a cyclin-dependent kinase
- PMID: 12417740
- PMCID: PMC137483
- DOI: 10.1073/pnas.232573899
Transcriptional activating regions target a cyclin-dependent kinase
Abstract
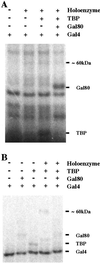
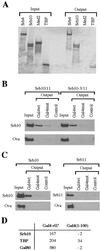
Several yeast activators are phosphorylated by SRB10, a cyclin-dependent kinase associated with the transcriptional machinery. Sites of phosphorylation are found outside the activating region in each case, and the modification has different physiological consequences in different cases. We show here that certain acidic transcriptional activating regions contact SRB10 as assayed both in vivo and in vitro. The interaction evidently positions each activator, as it activates transcription, so that it gets phosphorylated by SRB10, and thus a common mechanism targets disparate substrates to the kinase.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

