Antiepileptic drugs and apoptotic neurodegeneration in the developing brain
- PMID: 12417760
- PMCID: PMC137548
- DOI: 10.1073/pnas.222550499
Antiepileptic drugs and apoptotic neurodegeneration in the developing brain
Abstract
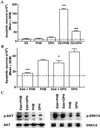
Epilepsy is the most common neurological disorder of young humans. Each year 150,000 children in the United States experience their first seizure. Antiepileptic drugs (AEDs), used to treat seizures in children, infants, and pregnant women, cause cognitive impairment, microcephaly, and birth defects. The cause of unwanted effects of therapy with AEDs is unknown. Here we reveal that phenytoin, phenobarbital, diazepam, clonazepam, vigabatrin, and valproate cause apoptotic neurodegeneration in the developing rat brain at plasma concentrations relevant for seizure control in humans. Neuronal death is associated with reduced expression of neurotrophins and decreased concentrations of survival-promoting proteins in the brain. beta-Estradiol, which stimulates pathways that are activated by neurotrophins, ameliorates AED-induced apoptotic neurodegeneration. Our findings present one possible mechanism to explain cognitive impairment and reduced brain mass associated with prenatal or postnatal exposure of humans to antiepileptic therapy.
Figures



References
-
- Hauser W. A. (1994) Epilepsia 35, Suppl. 2, S1-S6. - PubMed
-
- Taylor C. P. & Meldrum, B. S. (1995) Trends Pharmacol. Sci. 16, 309-316. - PubMed
-
- Meldrum B. S. (1996) Epilepsia 37, Suppl. 6, S4-S11. - PubMed
-
- Brodie M. J. & Dichter, M. A. (1996) N. Engl. J. Med. 334, 168-175. - PubMed
-
- Gidal B. E., Privitera, M. D., Sheth, R. D. & Gilman, J. T. (1999) Ann. Pharmacother. 33, 1277-1286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

