S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays
- PMID: 12417763
- PMCID: PMC137473
- DOI: 10.1073/pnas.232299399
S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays
Abstract
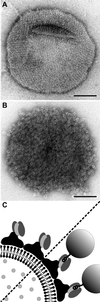
Biomolecular self-assembly can be used as a powerful tool for nanoscale engineering. In this paper, we describe the development of building blocks for nanobiotechnology, which are based on the fusion of streptavidin to a crystalline bacterial cell surface layer (S-layer) protein with the inherent ability to self-assemble into a monomolecular protein lattice. The fusion proteins and streptavidin were produced independently in Escherichia coli, isolated, and mixed to refold and purify heterotetramers of 1:3 stoichiometry. Self-assembled chimeric S-layers could be formed in suspension, on liposomes, on silicon wafers, and on accessory cell wall polymer containing cell wall fragments. The two-dimensional protein crystals displayed streptavidin in defined repetitive spacing, and they were capable of binding d-biotin and biotinylated proteins. Therefore, the chimeric S-layer can be used as a self-assembling nanopatterned molecular affinity matrix to arrange biotinylated compounds on a surface. In addition, it has application potential as a functional coat of liposomes.
Figures




References
-
- Winfree E., Liu, F., Wenzler, L. A. & Seeman, N. C. (1998) Nature 394, 539-544. - PubMed
-
- Seeman N. C. (1999) Trends Biotechnol. 17, 437-443. - PubMed
-
- Niemeyer C. M. (2000) Curr. Opin. Chem. Biol. 4, 609-618. - PubMed
-
- Sleytr U. B., Sára, M. & Pum, D. (2000) in Supramolecular Polymers, ed. Ciferri, A. (Dekker, New York), pp. 177–213.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

