Human CD8+ T cells do not require the polarization of lipid rafts for activation and proliferation
- PMID: 12419850
- PMCID: PMC137535
- DOI: 10.1073/pnas.232058599
Human CD8+ T cells do not require the polarization of lipid rafts for activation and proliferation
Abstract
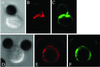
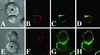
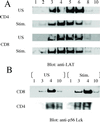
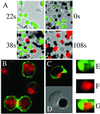
Lipid rafts are important signaling platforms in T cells. Little is known about their properties in human CD8(+) T cells. We studied polarization of lipid rafts by digital immunofluorescence microscopy in primary human T cells, using beads coated with anti-CD3 and anti-CD28 mAbs (CD3/28 beads). Unlike CD4(+) T cells, CD8(+) T cells did not polarize lipid rafts when stimulated with CD3/28 beads, when the anti-CD28 antibody was substituted with B7.2Ig, or if an anti-CD8 antibody was added to the CD3/28 beads. This phenomenon was also observed in human antigen-specific CD8(+) T cells. On stimulation with CD3/28 beads, the T cell antigen receptor clustered at the cell/bead contact area in both CD4(+) and CD8(+) T cells. Examination of lipid rafts isolated by sucrose density gradient centrifugation revealed the constitutive expression of p(56)Lck in the raft fractions of unstimulated CD8(+) T cells, whereas p(56)Lck was recruited to the raft fraction of CD4(+) T cells only after stimulation with CD3/28 beads. Stimulation with CD3/28 beads induced marked calcium flux, recruitment of PKC-theta and F-actin to the cell/bead contact site, and similar proliferation patterns in CD4(+) and CD8(+) T cells. Thus, polarization of lipid rafts is not essential for early signal transduction events or proliferation of human CD8(+) lymphocytes. It is possible that the lower stringency of CD8(+) T cell activation obviates a requirement for raft polarization.
Figures




References
-
- Monks C. R. F., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. (1998) Nature 395, 82-86. - PubMed
-
- Cherukuri A., Dykstra, M. & Pierce, S. K. (2000) Immunity 14, 657-660. - PubMed
-
- Viola A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. (1999) Science 283, 680-682. - PubMed
-
- Xavier R., Brennan, T., Li, Q., McCormack, C. & Seed, B. (1998) Immunity 8, 723-732. - PubMed
-
- Bi K., Tanaka, Y., Coudronniere, N., Sugie, K., Hong, S., van Stipdonk, M. J. B. & Altman, A. (2001) Nat. Immunol. 2, 556-562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

