Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals
- PMID: 12423310
- PMCID: PMC1782804
- DOI: 10.1046/j.1365-2567.2002.01469.x
Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals
Abstract
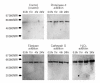
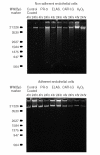
Thrombomodulin is a transmembranous glycoprotein of endothelial cells. In vitro it is a marker of endothelial cell injury. In vivo the levels of serum thrombomodulin are regarded as a parameter of activity in vasculitides. The latter are pathophysiologically determined by neutrophil-derived inflammation and endothelial cell injury caused by secretion of proteases and hydrogen peroxide. It was the objective of this study to determine whether thrombomodulin is only a late marker of advanced endothelial cell injury or whether it indicates also earlier stages of cell alterations. Over 24 hr endothelial cell cultures were incubated with hydrogen peroxide or the neutrophil proteases proteinase-3, elastase and cathepsin G. The time-dependent increase of thrombomodulin in the supernatant was determined by enzyme-linked immunosorbent assay and immunoblot. In addition the viability (eosin, tetrazolium dye assay), detachment (crystal-violet assay), and apoptosis (4',6-diamine-2'-phenylindole-dihydrochloride assay) of the respective endothelial cells were determined for adherent and non-adherent cells. A rapid thrombomodulin increase was found under all experimental conditions. The additional immunoblotting analysis showed the pattern of proteolytic cleavage caused by the protease reactivity. In case of hydrogen peroxide the thrombomodulin increase was closely correlated with the loss of cell viability and lysis. The incubation of endothelial cells with the different proteases resulted in a time-dependent detachment of primarily viable cells. In addition to cell necrosis apoptotic cell death was found in the subgroup of detached endothelial cells after prolonged incubation over 24 hr with proteinase-3 (23%), elastase (31%), and cathepsin G (19%). In contrast, still adhering cells did not show any signs of necrosis or apoptosis. In summary these studies confirm in vitro that soluble thrombomodulin is not only a parameter of advanced endothelial cell destruction itself but also in addition an early marker of initial endothelial cell membrane changes induced by neutrophil derived proteases and oxygen radicals.
Figures


Similar articles
-
Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro.J Lab Clin Med. 1994 Jun;123(6):874-81. J Lab Clin Med. 1994. PMID: 8201266
-
Interaction of endothelial cells and neutrophils in vitro: kinetics of thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1): implications for the relevance as serological disease activity markers in vasculitides.Clin Exp Immunol. 2000 Jan;119(1):250-4. doi: 10.1046/j.1365-2249.2000.01108.x. Clin Exp Immunol. 2000. PMID: 10606990 Free PMC article.
-
Modulation of human endothelial thrombomodulin by neutrophils and their release products.Am J Respir Crit Care Med. 1997 Jan;155(1):47-52. doi: 10.1164/ajrccm.155.1.9001288. Am J Respir Crit Care Med. 1997. PMID: 9001288
-
The role of neutrophil membrane glycoprotein 150 (Gp-150) in neutrophil-mediated endothelial cell injury in vitro.J Immunol. 1985 Jul;135(1):537-43. J Immunol. 1985. PMID: 3889157
-
Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock.Annu Rev Pharmacol Toxicol. 1993;33:71-90. doi: 10.1146/annurev.pa.33.040193.000443. Annu Rev Pharmacol Toxicol. 1993. PMID: 8494355 Review.
Cited by
-
Promising results of a clinical feasibility study: CIRBP as a potential biomarker in pediatric cardiac surgery.Front Cardiovasc Med. 2024 Feb 1;11:1247472. doi: 10.3389/fcvm.2024.1247472. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38361581 Free PMC article.
-
Potential Biomarkers for Early Diagnosis, Evaluation, and Prognosis of Sepsis-Induced Coagulopathy.Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296231195089. doi: 10.1177/10760296231195089. Clin Appl Thromb Hemost. 2023. PMID: 37605466 Free PMC article. Review.
-
The tripeptide feG inhibits leukocyte adhesion.J Inflamm (Lond). 2008 May 20;5:6. doi: 10.1186/1476-9255-5-6. J Inflamm (Lond). 2008. PMID: 18492254 Free PMC article.
-
Lymphangiogenesis in rat asthma model.Angiogenesis. 2017 Feb;20(1):73-84. doi: 10.1007/s10456-016-9529-2. Epub 2016 Oct 27. Angiogenesis. 2017. PMID: 27787629 Free PMC article.
-
Assessment of soluble thrombomodulin and soluble endoglin as endothelial dysfunction biomarkers in seriously ill surgical septic patients: correlation with organ dysfunction and disease severity.Eur J Trauma Emerg Surg. 2024 Jun;50(3):897-904. doi: 10.1007/s00068-023-02369-8. Epub 2023 Sep 23. Eur J Trauma Emerg Surg. 2024. PMID: 37741913
References
-
- Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–36. - PubMed
-
- Ishii H, Yama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost. 1991;65:618–23. - PubMed
-
- Sawada K, Yamamoto H, Yago H, Suehiro S. A simply assay to detect endothelial cell injury: measurement of released thrombomodulin from cells. Exp Mol Pathol. 1992;57:116–23. - PubMed
-
- Abe H, Okajima K, Okabe H, Takatsuki K, Binder BR. Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med. 1994;123:874–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

